-
Name
4,5-Dimethyl-1,3-dioxol-2-one
- EINECS 678-111-4
- CAS No. 37830-90-3
- Article Data13
- CAS DataBase
- Density 1.181 g/cm3
- Solubility
- Melting Point 78 °C
- Formula C5H6O3
- Boiling Point 117 °C at 760 mmHg
- Molecular Weight 114.101
- Flash Point 55.8 °C
- Transport Information
- Appearance white powder
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols
- Synonyms 4,5-Dimethyl-2-oxo-1,3-dioxolene;4,5-Dimethyldioxol-2-one;Dimethylvinylene carbonate;4,5-Dimethyl-1,3-dioxolen-2-one;
- PSA 43.35000
- LogP 0.84960
Synthetic route

-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

| Conditions | Yield |
|---|---|
| With acetic anhydride; zinc In toluene at 30 - 80℃; for 6h; Catalytic behavior; Reagent/catalyst; Temperature; Solvent; | 83% |
-
-
124-38-9
carbon dioxide

-
-
513-86-0, 52217-02-4
3-hydroxy-2-butanon

-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

| Conditions | Yield |
|---|---|
| Stage #1: carbon dioxide; 3-hydroxy-2-butanon With sodium carbonate; potassium hydrogencarbonate at 28 - 32℃; for 5h; Autoclave; Large scale; Green chemistry; Stage #2: With potassium methanolate; sodium ethanolate at 38 - 42℃; under 26252.6 Torr; Temperature; Reagent/catalyst; Pressure; Large scale; Green chemistry; | 80.7% |

| Conditions | Yield |
|---|---|
| In dichloromethane; N,N-dimethyl-aniline; toluene | 55% |
| In dichloromethane; N,N-dimethyl-aniline | 3.53 g (25%) |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
513-86-0, 52217-02-4
3-hydroxy-2-butanon

-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

| Conditions | Yield |
|---|---|
| Stage #1: bis(trichloromethyl) carbonate; 3-hydroxy-2-butanon With N,N-dimethyl-aniline In dichloromethane at 20℃; for 2h; Stage #2: at 160℃; for 4h; | 42% |

-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

| Conditions | Yield |
|---|---|
| at 160℃; for 4h; |
-
-
513-86-0, 52217-02-4
3-hydroxy-2-butanon

-
-
79-22-1
methyl chloroformate

-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-aniline at 15℃; for 5.5h; Temperature; | 450 g |
-
-
513-86-0, 52217-02-4
3-hydroxy-2-butanon

-
-
541-41-3
chloroformic acid ethyl ester

-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

| Conditions | Yield |
|---|---|
| In dichloromethane; N,N-dimethyl-aniline at 12℃; for 6h; Temperature; Solvent; | 850 g |
-
-
513-86-0, 52217-02-4
3-hydroxy-2-butanon

-
-
109-61-5
propoxycarbonyl chloride

-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

| Conditions | Yield |
|---|---|
| In dichloromethane; N,N-dimethyl-aniline at 20℃; for 0.5h; Temperature; Solvent; | 190 g |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
80715-22-6
4-bromomethyl-1,3-dioxa-5-methylcyclopentene-2-one

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In chloroform Reflux; Large scale; | 93.8% |
| With N-Bromosuccinimide; dibenzoyl peroxide In tetrachloromethane at 77℃; for 6h; | 92% |
| With bromine; pyridine hydrochloride-aluminum trichloride In acetone for 1.66667h; Reagent/catalyst; Wavelength; Solvent; Microwave irradiation; | 92% |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
80715-22-6
4-bromomethyl-1,3-dioxa-5-methylcyclopentene-2-one

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrachloromethane | 90% |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
80841-78-7
4-chloromethyl-5-methyl-1,3-dioxol-2-one

| Conditions | Yield |
|---|---|
| With chlorine In 1,2-dichloro-ethane for 0.5h; Solvent; Reagent/catalyst; Reflux; Molecular sieve; | 89.3% |
| With N-chloro-succinimide In tetrachloromethane for 80h; Ambient temperature; Irradiation; | 9.2% |
| With chlorine; copper 1.) dichloromethane, 90 min, reflux, 2.) dichloromethane, 2 h, reflux; Yield given. Multistep reaction; | |
| With sulfuryl dichloride In dichloromethane | |
| With N-chloro-succinimide; dibenzoyl peroxide at 90℃; for 5.5h; Temperature; Solvent; Large scale; | 326 g |

| Conditions | Yield |
|---|---|
| With tris(acetonitrile)pentamethylcyclopentadienylrhodium(III) hexafluoroantimonate In 1,2-dichloro-ethane at 80℃; for 20h; Inert atmosphere; | 89.3% |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
95579-71-8
4-chloro-4-methyl-5-methylene-1,3-dioxolane-2-one

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride In dichloromethane for 0.5h; Heating; | 75% |
| With sulfuryl dichloride In dichloromethane | |
| With thionyl chloride In dichloromethane for 4.5h; Solvent; Reflux; | |
| With sulfuryl dichloride In dichloromethane at 40℃; for 2h; |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
62458-19-9
4,5-di(bromomethyl)-2-oxo-1,3-dioxole

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In benzene at 110℃; for 2h; Inert atmosphere; | 66% |
| With N-Bromosuccinimide; azobisisobutyronitrile In tetrachloromethane | |
| With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In benzene for 1h; Reflux; |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
34241-39-9
azobisisobutyronitrile

-
-
80715-22-6
4-bromomethyl-1,3-dioxa-5-methylcyclopentene-2-one

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrachloromethane | 48% |
| With N-Bromosuccinimide In tetrachloromethane |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
A
-
80841-78-7
4-chloromethyl-5-methyl-1,3-dioxol-2-one

-
B
-
95579-71-8
4-chloro-4-methyl-5-methylene-1,3-dioxolane-2-one

-
C
-
129482-56-0
4,5-dimethyl-4,5-dichloro-1,3-dioxolan-2-one

| Conditions | Yield |
|---|---|
| With chlorine In dichloromethane at 43 - 45℃; Product distribution; further solvents; | A 1.4 % Chromat. B 87.5 % Chromat. C n/a |

-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
100165-57-9
(5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl trans-4-<<(tert-butoxycarbonyl)amino>methyl>cyclohexanecarboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-bromosuccinimide, α,α-azobis(isobutyronitrile) / CCl4 2: 41 percent / K2CO3, (C4H9)4NBr (QBr) / trichloroethene / 20 h / 60 °C View Scheme |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
100165-54-6
(5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl trans-4-(aminomethyl)cyclohexanecarboxylate hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: N-bromosuccinimide, α,α-azobis(isobutyronitrile) / CCl4 2: 41 percent / K2CO3, (C4H9)4NBr (QBr) / trichloroethene / 20 h / 60 °C 3: 70 percent / HCl(g) / ethyl acetate / 2.5 h / 0 - 20 °C View Scheme |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
100165-55-7
C21H32N2O7*2ClH

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: N-bromosuccinimide, α,α-azobis(isobutyronitrile) / CCl4 2: K2CO3, (C4H9)4NBr (QBr) / trichloroethene / 20 h / 60 °C 3: HCl(g) / ethyl acetate / 2.5 h / 0 - 20 °C View Scheme |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-bromosuccinimide, α,α-azobis(isobutyronitrile) / CCl4 2: K2CO3, (C4H9)4NBr (QBr) / trichloroethene / 20 h / 60 °C View Scheme |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
80841-79-8
(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl iodide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 80.8 percent / sodium bicarbonate, α,α'-azobisisobutyronitrile, Br2 / CCl4 / 1.08 h / 70 °C 2: 92 percent / potassium iodide / acetone / Ambient temperature View Scheme |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 80.8 percent / sodium bicarbonate, α,α'-azobisisobutyronitrile, Br2 / CCl4 / 1.08 h / 70 °C 2: 1.) potassium bicarbonate / 1.) DMF, 0 deg C, 3 h; 2.) 0 deg C, 3 h View Scheme |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
80734-02-7
ampicillin (5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 80.8 percent / sodium bicarbonate, α,α'-azobisisobutyronitrile, Br2 / CCl4 / 1.08 h / 70 °C 2: 1.) potassium bicarbonate / 1.) DMF, 0 deg C, 3 h; 2.) 0 deg C, 3 h 3: 60 percent / 1N HCl / acetonitrile / 0.5 h / 0 - 5 °C / pH=2.0 View Scheme |
-
-
128-08-5
N-Bromosuccinimide

-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
80715-22-6
4-bromomethyl-1,3-dioxa-5-methylcyclopentene-2-one

| Conditions | Yield |
|---|---|
| 2,2'-azobis(isobutyronitrile) In benzene at 20℃; for 0.5h; Heating / reflux; |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
91526-18-0
4-hydroxymethyl-5-methyl-[1,3]dioxol-2-one

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide In 1,4-dioxane | |
| With selenium(IV) oxide In 1,4-dioxane | |
| With selenium(IV) oxide In 1,4-dioxane for 1h; Heating / reflux; |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide; dibenzoyl peroxide / tetrachloromethane / 2 h / Reflux 2: sodium hydrogencarbonate / N,N-dimethyl-formamide / 20 °C View Scheme |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
123447-62-1
prulifloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide / chloroform / Reflux; Large scale 2: potassium hydrogencarbonate / N,N-dimethyl-formamide / 5 h / 0 °C / Large scale View Scheme |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) / tetrachloromethane / 2 h / 80 °C 2: potassium carbonate / acetone / 24 h / 55 °C View Scheme |
-
-
37830-90-3
4,5-Dimethyl-1,3-dioxole-2-one

-
-
1026789-40-1
(S)-3-(tert-butoxycarbonylamino-methyl)-5-methyl-hexanoic acid 5-methyl-2-oxo-[1,3]dioxol-4-ylmethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-Bromosuccinimide / 2,2'-azobis(isobutyronitrile) / tetrachloromethane 2: caesium carbonate / methanol / 2 h / 20 °C View Scheme |
4,5-Dimethyl-1,3-dioxol-2-one Specification
The 4,5-Dimethyl-1,3-dioxol-2-one, with the CAS registry number 37830-90-3, is also known as 1,3-Dioxol-2-one,4,5-dimethyl-. It belongs to the product categories of Oxygen Cyclic Compounds; Fluorobenzene; Agricultural Chemicals(bactericide); Pharmaceutical Intermediates. This chemical's molecular formula is C5H6O3 and molecular weight is 114.09934. Its IUPAC name is called 4,5-dimethyl-1,3-dioxol-2-one. What's more, this chemical is used as an intermediates of the Antihypertensive drugs-Olmesartan medoxomil.
Physical properties of 4,5-Dimethyl-1,3-dioxol-2-one: (1)ACD/LogP: 1.45; (2)ACD/LogD (pH 5.5): 1.45; (3)ACD/LogD (pH 7.4): 1.45; (4)ACD/BCF (pH 5.5): 7.5; (5)ACD/BCF (pH 7.4): 7.5; (6)ACD/KOC (pH 5.5): 147.2; (7)ACD/KOC (pH 7.4): 147.2; (8)#H bond acceptors: 3; (9)Index of Refraction: 1.454; (10)Molar Refractivity: 26.18 cm3; (11)Molar Volume: 96.5 cm3; (12)Surface Tension: 30.3 dyne/cm; (13)Density: 1.181 g/cm3; (14)Flash Point: 55.8 °C; (15)Enthalpy of Vaporization: 35.53 kJ/mol; (16)Boiling Point: 117 °C at 760 mmHg; (17)Vapour Pressure: 17.8 mmHg at 25°C.
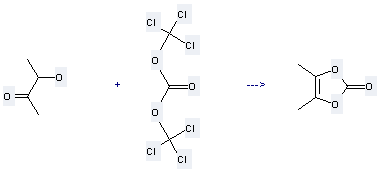
Preparation 4,5-Dimethyl-1,3-dioxol-2-one: this chemical can be prepared by 3-hydroxy-butan-2-one and carbonic acid bis-trichloromethyl ester. This reaction will need reagent N,N-dimethylaniline and solvent CH2Cl2. The yield is about 42%.
Uses of 4,5-Dimethyl-1,3-dioxol-2-one: it can be used to produce 4-chloro-4-methyl-5-methylene-1,3-dioxolan-2-one by heating. This reaction will need reagent sulfuryl chloride and solvent CH2Cl2 with reaction time of 30 min. The yield is about 75%.

When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC1=C(OC(=O)O1)C
(2)InChI: InChI=1S/C5H6O3/c1-3-4(2)8-5(6)7-3/h1-2H3
(3)InChIKey: QYIOFABFKUOIBV-UHFFFAOYSA-N
Related Products
- 4-06-00-02342 (Beilstein Handbook Reference)
- 4,10-Ace-1,2-benzanthracene
- 4,10-Dioxatricyclo[5.2.1.0(2,6)]decan-8-en-3-one
- 4-(1,1,2,2-Tetrafluoroethoxy)benzoicacid
- 4-(1,1,2,2-Tetrafluoroethoxy)chlorobenzene
- 4-(1,1,2,2-Tetrafluoroethoxy)nitrobenzene
- 4-(1,1,2,2-Tetrafluoroethoxy)toluene
- 4-(1,1-Difluoropropan-2-yl)benzene-1-sulfonyl chloride
- 4-(1,1-Dioxothiazolidin-2-yl)benzoate
- 4′-(1,2,3,4-TETRAHYDRO-4-(4-HYDROXY-2-OXO-2H-1-BENZOPYRAN-3-YL)-2-NAPHTHALENYL)(1,1′-BIPHENYL)-4-CARBONITRILE, cis-
- 37831-62-2
- 37831-70-2
- 3783-61-7
- 37836-80-9
- 37839-01-3
- 37839-81-9
- 3784-03-0
- 37843-14-4
- 378-44-9
- 37845-05-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View