-
Name
4-Amino-3,5-dichloro-alpha-bromoacetophenone
- EINECS 253-367-6
- CAS No. 37148-47-3
- Article Data18
- CAS DataBase
- Density 1.764 g/cm3
- Solubility
- Melting Point 142-145 °C
- Formula C8H6BrCl2NO
- Boiling Point 396.4 °C at 760 mmHg
- Molecular Weight 282.952
- Flash Point 193.6 °C
- Transport Information
- Appearance Yellow solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1-(4-Amino-3,5-dichlorophenyl)-2-bromoethanone;4-Amino-w-bromo-3,5-dichloroacetophenone;
- PSA 43.09000
- LogP 3.73440
Synthetic route
-
-
37148-48-4
1-(4-amino-3,5-dichlorophenyl)-1-ethanone

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| With bromine at 20℃; for 3h; | 95.2% |
| Stage #1: 1-(4-amino-3,5-dichlorophenyl)-1-ethanone With bromine In chloroform for 0.5h; Reflux; Inert atmosphere; Stage #2: With triethylamine; phosphonic acid diethyl ester In tetrahydrofuran at 0 - 20℃; for 0.166667h; Inert atmosphere; | 89% |
| Stage #1: 1-(4-amino-3,5-dichlorophenyl)-1-ethanone With bromine In tetrahydrofuran; chloroform for 0.416667h; Reflux; Stage #2: With diethyl phosphite; triethylamine In tetrahydrofuran at 20℃; for 0.166667h; | 89% |
-
-
608-31-1
2,6-Dichloroaniline

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: iron(III) chloride / chloroform / 6 h / 0 - 20 °C 2: bromine / 3 h / 20 °C View Scheme |
-
-
99-92-3
4-Aminoacetophenone

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-chloro-succinimide / acetonitrile / 4.5 h / 20 °C 2: copper(ll) bromide / ethyl acetate; chloroform; ethanol / 2 h / Reflux View Scheme |
-
-
6045-08-5
1,1,1,3,3,3-hexadeuterio-2-(trideuteriomethyl)propan-2-amine

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 6h; Solvent; | 96% |
| With N-ethyl-N,N-diisopropylamine In chloroform for 3h; Reflux; | 72% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
124-68-5
2-Amino-2-methyl-1-propanol

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 2h; | 91% |
-
-
27646-80-6
N-Methyl-2-amino-2-methyl-1-propanol

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
1242652-49-8
2-(4-amino-3,5-dichlorophenyl)-4,5,5-trimethylmorpholin-2-ol

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 2h; | 91% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
10312-55-7
3-aminoterephthalic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 1h; | 90% |
-
-
32187-15-8
thioanthranilic acid

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 1h; | 90% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
18197-26-7
sodium diformamide

| Conditions | Yield |
|---|---|
| In acetonitrile at 70℃; for 2h; | 88.1% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
85743-02-8
2-amino-benzene-1,4-dicarboxylic acid,4-methyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In N,N-dimethyl-formamide at 20℃; | 82% |
-
-
5699-40-1
acetylguanidine

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| In acetonitrile at 100℃; for 3h; | 81% |
| In acetonitrile at 100℃; for 3h; | 81% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
75-31-0
isopropylamine

| Conditions | Yield |
|---|---|
| at 20℃; for 6h; | 80.2% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| for 3h; Reflux; | 79.5% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
118-92-3
anthranilic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In N,N-dimethyl-formamide 1.) 90 - 100 deg C, 2.) 25 - 28 deg C, 1 h; | 79% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
17356-08-0
thiourea

| Conditions | Yield |
|---|---|
| In water at 20℃; for 2h; | 76% |
| In water at 20℃; for 2h; | 76% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
56-04-2
2-mercapto-6-methylpyrimidin-4(3H)-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-mercapto-6-methylpyrimidin-4(3H)-one With potassium hydroxide In methanol at 22 - 25℃; under 760.014 Torr; for 0.25h; Inert atmosphere; Stage #2: 1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one In methanol at 22 - 25℃; under 760.014 Torr; for 12h; Inert atmosphere; | 71% |
-
-
5434-20-8
3-aminophthalic acid

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 2h; | 68% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
141-90-2
2-mercaptopyrimidin-4(3H)-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-mercaptopyrimidin-4(3H)-one With potassium hydroxide In methanol at 22 - 25℃; under 760.014 Torr; for 0.25h; Inert atmosphere; Stage #2: 1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one In methanol at 22 - 25℃; under 760.014 Torr; for 12h; Inert atmosphere; | 68% |
-
-
6045-08-5
1,1,1,3,3,3-hexadeuterio-2-(trideuteriomethyl)propan-2-amine

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,3,3,3-hexadeuterio-2-(trideuteriomethyl)propan-2-amine; 1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one With tetrabutylammomium bromide In dichloromethane at 30℃; for 5h; Stage #2: With sodium tetrahydroborate In dichloromethane at 20℃; for 5h; | 57.8% |
-
-
2306-33-4
monoethyl phthalate

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| With triethylamine In acetone for 0.5h; Heating; | 52% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
372-09-8
cyanoacetic acid

-
-
1078715-64-6
C11H8Cl2N2O3

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide at 0℃; for 5h; | 51% |
-
-
4442-59-5
(2,3-dihydrobenzo[1,4]dioxin-2-ylmethyl)amine

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| In methanol for 6h; Heating; | 50% |
-
-
1071-46-1
hydrogen ethyl malonate

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
1078715-65-7
C13H13Cl2NO5

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl-formamide at 0℃; for 1h; | 43% |
-
-
55432-59-2
<(β,β,β,β',β',β'-D6)isopropyl>amine

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| In chloroform at 63℃; for 5h; Sealed tube; | 37.4% |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
75-64-9
tert-butylamine

-
-
37148-27-9
clenbuterol

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one; tert-butylamine In tetrahydrofuran; ethanol at 0 - 20℃; for 4h; Inert atmosphere; Stage #2: With potassium borohydride In tetrahydrofuran; ethanol for 2h; Cooling with ice; Stage #3: With methanol In tetrahydrofuran; ethanol at 20℃; for 16h; | 35% |
-
-
110-91-8
morpholine

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

-
-
37163-03-4
1-(4-amino-3,5-dichloro-phenyl)-2-morpholin-4-yl-ethanol

| Conditions | Yield |
|---|---|
| (i), (ii) NaBH4; Multistep reaction; |
-
-
6045-08-5
1,1,1,3,3,3-hexadeuterio-2-(trideuteriomethyl)propan-2-amine

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| In chloroform for 2h; Heating; |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 52 percent / triethylamine / acetone / 0.5 h / Heating 2: 82 percent / KOH / 1-methyl-pyrrolidin-2-one / 2 h / 45 °C View Scheme |
-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 50 percent / methanol / 6 h / Heating 2: 58 percent / sodium borohydride / methanol; H2O / 1.) 0 deg C; 2.) rt, 1 h View Scheme |

-
-
37148-47-3
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one

| Conditions | Yield |
|---|---|
| With sodium borohydrid; ammonia In methanol; chloroform; water |
4-Amino-3,5-dichloro-alpha-bromoacetophenone Specification
The Ethanone,1-(4-amino-3,5-dichlorophenyl)-2-bromo-, with the CAS registry number 37148-47-3 and EINECS registry number 253-367-6, has the systematic name of 1-(4-amino-3,5-dichlorophenyl)-2-bromoethanone. It is a kind of yellow solid, and belongs to the following product categories: Aromatics Compounds; Aromatics. And the molecular formula of the chemical is C8H6BrCl2NO.
The characteristics of Ethanone,1-(4-amino-3,5-dichlorophenyl)-2-bromo- are as followings: (1)ACD/LogP: 3.39; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.39; (4)ACD/LogD (pH 7.4): 3.39; (5)ACD/BCF (pH 5.5): 223.64; (6)ACD/BCF (pH 7.4): 223.64; (7)ACD/KOC (pH 5.5): 1672.69; (8)ACD/KOC (pH 7.4): 1672.69; (9)#H bond acceptors: 2; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 20.31 Å2; (13)Index of Refraction: 1.643; (14)Molar Refractivity: 58.03 cm3; (15)Molar Volume: 160.3 cm3; (16)Polarizability: 23×10-24cm3; (17)Surface Tension: 57.8 dyne/cm; (18)Density: 1.764 g/cm3; (19)Flash Point: 193.6 °C; (20)Enthalpy of Vaporization: 64.67 kJ/mol; (21)Boiling Point: 396.4 °C at 760 mmHg; (22)Vapour Pressure: 1.71E-06 mmHg at 25°C.
Uses of Ethanone,1-(4-amino-3,5-dichlorophenyl)-2-bromo-: It can react with 2-amino-benzoic acid to produce 2-amino-benzoic acid 2-(4-amino-3,5-dichloro-phenyl)-2-oxo-ethyl ester. This reaction will need reagent NaHCO3, and the menstruum dimethylformamide. And the yield is about 79%.
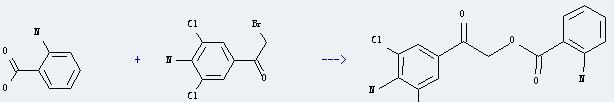
Addtionally, the following datas could be converted into the molecular structure:
(1)SMILES: Clc1cc(cc(Cl)c1N)C(=O)CBr
(2)InChI: InChI=1/C8H6BrCl2NO/c9-3-7(13)4-1-5(10)8(12)6(11)2-4/h1-2H,3,12H2
(3)InChIKey: ATKJJUFAWYSFID-UHFFFAOYAL
Related Products
- 4-Amino-3-(3,4-dichlorophenyl)-1-butanol
- 4-Amino-3-(trifluoromethoxy)benzoic acid
- 4-Amino-3-(trifluoromethoxy)benzonitrile
- 4-Amino-3-(trifluoromethyl)phenol
- 4-Amino-3,4-dihydro-2(1H)-quinolinone
- 4-Amino-3,5-dibromobenzoic acid
- 4-Amino-3,5-dibromopyridine
- 4-Amino-3,5-dichloro-2,6-difluoropyridine
- 4-Amino-3,5-dichloro-alpha-bromoacetophenone
- 4-Amino-3,5-dichlorobenzoic acid
- 37148-48-4
- 3715-16-0
- 3715-29-5
- 37153-52-9
- 37154-85-1
- 37155-50-3
- 37156-84-6
- 37159-60-7
- 371-62-0
- 37167-59-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View