-
Name
4-Aminosalicylic acid
- EINECS 200-613-5
- CAS No. 65-49-6
- Article Data63
- CAS DataBase
- Density 1.491 g/cm3
- Solubility water: 2 g/L (20 °C )
- Melting Point 135-145 °C(lit.)
- Formula C7H7NO3
- Boiling Point 380.8 °C at 760 mmHg
- Molecular Weight 153.137
- Flash Point 184.1 °C
- Transport Information UN 1789 8/PG 3
- Appearance beige powder
- Safety 26-37/39-45-53-36
- Risk Codes 22-36-36/37/38-45
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi,
Xi, T
T
- Synonyms Salicylicacid, 4-amino- (8CI);2-Hydroxy-4-aminobenzoic acid;4-Aminosalicylicacid;Entepas;Gabbropas;Osacyl;PAS (acid);Pamisyl;Para-Pas;Paramycin;Parasal;Parasalindon;Pasnodia;Propasa;Sanipirol-4;
- PSA 83.55000
- LogP 1.25380
Synthetic route

| Conditions | Yield |
|---|---|
| With aminomethyl polystyrene resin formic acid salt; zinc In methanol at 20℃; for 2h; | 96% |
| With ammoniummethyl polystyrene resin formate; palladium on activated charcoal In methanol at 20℃; for 5h; | 95% |
| With polymer-supported formate; magnesium In methanol at 20℃; for 3h; | 94% |
-
-
43134-76-5
1-amino-3-acetylamino-6-carboxy-benzene

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium nitrite Eintragen der Reaktionsloesung in warme Schwefelsaeure; |

| Conditions | Yield |
|---|---|
| With carbon dioxide; boric acid; potassium hydrogencarbonate at 95℃; weiteres Reagens: H2O; | |
| With carbon dioxide; potassium hydrogencarbonate; glycerol at 130℃; | |
| With potassium hydroxide; sodium dithionite; carbon dioxide at 100℃; under 73550.8 Torr; | |
| With carbon dioxide; potassium carbonate at 180℃; | |
| With salicylic acid decarboxylase from Trichosporon moniliiforme; potassium hydrogencarbonate at 30℃; for 24h; pH=8.5; aq. phosphate buffer; Enzymatic reaction; regioselective reaction; |
-
-
591-27-5
m-Hydroxyaniline

-
A
-
15540-79-1
4-amino-6-hydroxyisophthalic acid

-
B
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| With carbon dioxide; potassium hydrogencarbonate |

| Conditions | Yield |
|---|---|
| With ammonium carbonate at 215℃; under 154457 Torr; anschliessend Erhitzen mit Na2CO3 und CO2 auf 125grad/140 at; | |
| With ammonium hydroxide; boric acid at 200℃; under 33097.9 Torr; anschliessend Erhitzen mit NaHCO3 und CO2 auf 110grad/20 at; |

| Conditions | Yield |
|---|---|
| at 110℃; |

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| With water; nickel Hydrogenation; |

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 260℃; |

| Conditions | Yield |
|---|---|
| With carbon dioxide at 85 - 90℃; |

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| With water; nickel Hydrogenation; |

| Conditions | Yield |
|---|---|
| at 200℃; under 37503 Torr; for 1h; Kolbe-Shmitt reaction; | 80.0 % Chromat. |

| Conditions | Yield |
|---|---|
| at 200℃; under 37503 Torr; for 1h; Kolbe-Shmitt reaction; | 80.0 % Chromat. |

| Conditions | Yield |
|---|---|
| at 250℃; under 37503 Torr; for 1h; Kolbe-Shmitt reaction; | A 5.9 % Chromat. B 50.6 % Chromat. |

-
-
39835-14-8
2-hydroxy-4-nitro-benzonitrile

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aqueous sulfuric acid; acetic acid / Siedetemperatur 2: iron (II)-sulfate; sodium carbonate; water View Scheme | |
| Multi-step reaction with 2 steps 1: aqueous hydrochloric acid / 160 °C / Reinigung ueber das Barium-Salz 2: Raney nickel; sodium-<4-nitro-2-hydroxy benzoate>; water / Hydrogenation View Scheme |

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| With rat fecal matter at 37℃; pH=7.4; Kinetics; aq. phosphate buffer; |
-
-
1122102-49-1
C18H16N3O4(1-)*Na(1+)

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| With rat fecal matter at 37℃; pH=7.4; Kinetics; aq. phosphate buffer; |

| Conditions | Yield |
|---|---|
| With recombinant Escherichia coli cells expressing salicylic acid decarboxylase F195Y at 30℃; pH=6; Kinetics; Reagent/catalyst; Kolbe-Schmitt reaction; aq. phosphate buffer; Enzymatic reaction; | |
| With Trichosporon moniliiforme WU-0401 salicylic acid decarboxylase at 30℃; for 1h; pH=7; enzymatic Kolbe-Schmitt reaction; aq. phosphate buffer; Enzymatic reaction; regioselective reaction; | |
| With salicylic acid decarboxylase Y64T-F195Y mutant In aq. phosphate buffer at 30℃; for 2h; pH=6; Kinetics; Reagent/catalyst; Time; Kolbe-Schmidt Synthesis; | |
| With salicylic acid decarboxylase from Trichosporon moniliiforme In aq. phosphate buffer at 30℃; for 24h; Kolbe-Schmidt Synthesis; Enzymatic reaction; regioselective reaction; |
-
-
17980-39-1
1,1,3,3,5,5-hexamethyl-1,5-divinyltrisiloxane

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| In toluene | |
| In toluene | |
| In toluene |
-
-
6018-18-4
4-amino-2-hydroxy-benzoic acid ; hydrochloride

-
-
65944-71-0
cytosinium 4-aminosalicylate

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| In ethanol Milling; |

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
65-49-6
4-Aminosalicylic acid

-
-
4136-97-4
methyl 4-aminosalicylate

| Conditions | Yield |
|---|---|
| In diethyl ether at 25℃; for 0.333333h; | 100% |
| With diethyl ether |

| Conditions | Yield |
|---|---|
| In formic acid; diethyl ether | 99% |
-
-
65-49-6
4-Aminosalicylic acid

-
-
501-53-1
benzyl chloroformate

-
-
6935-15-5
4‐{[(benzyloxy)carbonyl]amino}‐2‐hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In methanol at 20℃; for 2.5h; | 98% |
| With sodium hydrogencarbonate In methanol at 20℃; for 24h; | 71% |
| With sodium hydrogencarbonate In tetrahydrofuran at 20℃; for 16h; | 61% |
| With sodium hydrogencarbonate In tetrahydrofuran at 20℃; for 16h; | |
| With sodium carbonate In tetrahydrofuran; water at 20℃; for 1h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| In ethanol; water at 75℃; for 0.5h; Product distribution / selectivity; | 98% |
| In ethanol at 80℃; for 0.333333h; Product distribution / selectivity; | 95% |

-
-
65-49-6
4-Aminosalicylic acid

-
-
66761-27-1
4-carboxy-3-hydroxyphenyl azide

| Conditions | Yield |
|---|---|
| Stage #1: 4-Aminosalicylic acid With sulfuric acid; sodium nitrite In water at 0℃; for 1h; Stage #2: With sodium azide In water at 0℃; for 1h; | 97.6% |
| Stage #1: 4-Aminosalicylic acid With sulfuric acid; sodium nitrite In water at 0℃; for 0.333333h; Stage #2: With sodium azide; urea In water at 0℃; | 86% |
| Stage #1: 4-Aminosalicylic acid With hydrogenchloride; sodium nitrite In water at 0℃; for 0.25h; Stage #2: With sodium azide In water at 0℃; for 1h; | 57% |

| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate In 1-methyl-pyrrolidin-2-one; toluene at 100℃; for 24h; | 96% |

| Conditions | Yield |
|---|---|
| With methanol; sulfuric acid at 0 - 80℃; Large scale reaction; | 95.1% |

| Conditions | Yield |
|---|---|
| In acetone for 24h; Cooling with ice; | 95% |
| With sodium hydroxide In water at 60℃; for 4h; pH=6-7; | 91% |
| In acetone for 6h; | 91% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; for 10h; Reflux; | 95% |
| With sulfuric acid at 0 - 20℃; for 6h; Reflux; | 93% |
| With sulfuric acid at 0℃; for 6h; Reflux; | 93% |

| Conditions | Yield |
|---|---|
| In methanol for 1h; Reflux; | 94% |
| In methanol at 20℃; for 24h; |

| Conditions | Yield |
|---|---|
| In methanol for 1h; Reflux; | 94% |

| Conditions | Yield |
|---|---|
| With (2,6-dichlorophenyl)bis(2,3,5,6-tetrafluorophenyl)borane; hydrogen In tetrahydrofuran at 100℃; under 60804.1 Torr; for 15h; Sealed tube; Molecular sieve; Autoclave; Green chemistry; | 94% |
-
-
65-49-6
4-Aminosalicylic acid

-
-
84473-96-1
4-amino-3,5-dichloro-2-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In 1,4-dioxane at 80 - 85℃; for 4h; | 94% |
-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In 1,2-dichloro-ethane at 80 - 85℃; for 4h; | 93% |
| With bromine; acetic acid |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
65-49-6
4-Aminosalicylic acid

-
-
184033-42-9
4-((tert-butoxycarbonyl)amino)-2-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water Schlenk technique; Inert atmosphere; | 93% |
| With triethylamine In tetrahydrofuran; methanol at 50℃; for 72h; | 91% |
| Stage #1: di-tert-butyl dicarbonate; 4-Aminosalicylic acid With triethylamine In 1,4-dioxane; water at 0 - 20℃; for 24.5h; Stage #2: With hydrogenchloride In water | 80% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone at 25℃; for 5.5h; Concentration; Reagent/catalyst; Large scale; | 92.1% |
| With sodium hydroxide In acetone at 20℃; for 6h; Time; | 92.82% |
| With tetrabutylammomium bromide; potassium hydroxide In acetone at 20 - 30℃; for 1.33333h; | 84% |
-
-
17422-74-1
3-Formylchromone

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 3h; Heating; | 92% |
-
-
24677-78-9
2,3-dihydroxybenzaldehyde

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| In methanol for 1h; Reflux; | 92% |
| In methanol at 20℃; for 24h; |

| Conditions | Yield |
|---|---|
| With acetic anhydride In ethanol | 92% |
| With acetic anhydride In ethanol | 72.3 g (74.15%) |
-
-
34619-03-9
tert-butyldicarbonate

-
-
65-49-6
4-Aminosalicylic acid

-
-
184033-42-9
4-((tert-butoxycarbonyl)amino)-2-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-Aminosalicylic acid With sodium hydroxide In ethanol; water at 5 - 10℃; Stage #2: tert-butyldicarbonate In ethanol; water at 5 - 10℃; Stage #3: In ethanol; water | 92% |
-
-
1394140-47-6
4-(1H-benzo[d]imidazol-2-yl)-4-oxobutane hydrazide

-
-
65-49-6
4-Aminosalicylic acid

-
-
1394140-28-3
3-(5-(4-amino-2-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)-1-(1H-benzo[d]imidazol-2-yl)propan-1-one

| Conditions | Yield |
|---|---|
| With trichlorophosphate Microwave irradiation; | 92% |
-
-
23911-25-3
ethylenediaminetetraacetic dianhydride

-
-
65-49-6
4-Aminosalicylic acid

-
-
41314-68-5
4,4′-((2,2′-(ethane-1,2-diylbis((carboxymethyl) azanediyl)) bis(acetyl))bis(azanediyl))bis(2-hydroxybenzoic acid)

| Conditions | Yield |
|---|---|
| With pyridine In N,N-dimethyl-formamide at 30℃; for 24h; Inert atmosphere; | 92% |
-
-
22065-57-2
ethyl 2-phenyldiazoacetate

-
-
65-49-6
4-Aminosalicylic acid

| Conditions | Yield |
|---|---|
| With C43H37Br2CuN3P2(1+)*ClO4(1-) In methanol at 0 - 20℃; for 3h; Schlenk technique; Inert atmosphere; chemoselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| In water Tl2CO3 added to water and org. compd., boiled for 2 h with stirring; soln. slowly concd.; | 90% |
-
-
88258-42-8
4-methoxy-6-hydroxybenzofuran-5-carboxylic acid

-
-
65-49-6
4-Aminosalicylic acid

-
-
1248345-36-9
7-[2-(4-carboxy-3-hydroxyphenyl)azo]-6-hydroxy-4-methoxy-benzofuran-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-Aminosalicylic acid With hydrogenchloride; sodium nitrite In water at 0 - 10℃; Stage #2: 6-hydroxy-4-methoxybenzofuran-5-carboxylic acid With sodium hydroxide In water at 5℃; | 90% |

| Conditions | Yield |
|---|---|
| In acetone at 20℃; | 90% |

| Conditions | Yield |
|---|---|
| With potassium phosphate tribasic trihydrate In N,N-dimethyl-formamide at 100℃; under 760.051 Torr; for 6h; Green chemistry; | 90% |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 40℃; for 3.5h; Molecular sieve; | 90% |
-
-
50-00-0
formaldehyd

-
-
65-49-6
4-Aminosalicylic acid

-
-
1133108-20-9
4-(1,3,5-dithiazinan-5-yl)-2-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With hydrogen sulfide In water at 20℃; | 89% |
4-Aminosalicylic acid History
4-Aminosalicylic acid Consensus Reports
4-Aminosalicylic acid Specification
The 4-Aminosalicylic acid, with the CAS registry number 65-49-6, is also known as Aminosalicylic Acid. It belongs to the product categories of Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts; Organic Acids; Hematology and Histology; Histology Special Stains; Electrophoresis of Glycans Stains and Dyes; Glycan Labeling and Analysis; Glycobiology; S; Stains & Dyes, A to; Amines; Aromatics; Intermediates & Fine Chemicals; Pharmaceuticals. Its EINECS registry number is 200-613-5. This chemical's molecular formula is C7H7NO3 and molecular weight is 153.14. What's more, both its IUPAC name and systematic name are the same which is called 4-Amino-2-hydroxybenzoic acid. It should be stored in a sealed place and avoid light. It is manufactured by the carboxylation of 3-aminophenol. This chemical is an antibiotic used to treat tuberculosis.
Physical properties about 4-Aminosalicylic acid are: (1)ACD/LogP: 1.242; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.36; (4)ACD/LogD (pH 7.4): -1.89; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 4; (10)#H bond donors: 4; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 83.55 Å2; (13)Index of Refraction: 1.691; (14)Molar Refractivity: 39.301 cm3; (15)Molar Volume: 102.673 cm3; (16)Polarizability: 15.58×10-24 cm3; (17)Surface Tension: 83.37 dyne/cm; (18)Density: 1.492 g/cm3; (19)Flash Point: 184.076 °C; (20)Enthalpy of Vaporization: 66.344 kJ/mol; (21)Boiling Point: 380.758 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25 °C.
Uses of This reaction needs solvent diethyl ether at temperature of 25 °C. The reaction time is 25 min. The yield is 100 %.
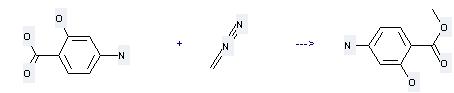
When you are dealing with this chemical, you should be very careful. This chemical may cause damage to health at low levels and it may cause inflammation to the skin or other mucous membranes. In addition, it is toxic by inhalation, in contact with skin and if swallowed. The product is irritating to eyes, respiratory system and skin. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell you should seek medical advice immediately.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(O)c1ccc(cc1O)N
(2) InChI: InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11)
(3) InChIKey: WUBBRNOQWQTFEX-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 4250mg/kg (4250mg/kg) | Zeitschrift fuer Naturforschung, Teil B: Anorganische Chemie, Organische Chemie, Biochemie, Biophysik, Biologie. Vol. 6B, Pg. 183, 1951. | |
| mouse | LD50 | intravenous | 3898mg/kg (3898mg/kg) | Antibiotiki. Vol. 18, Pg. 249, 1973. | |
| mouse | LD50 | oral | 4gm/kg (4000mg/kg) | Journal of Pharmacy and Pharmacology. Vol. 2, Pg. 764, 1950. | |
| mouse | LD50 | subcutaneous | 4gm/kg (4000mg/kg) | Journal of Pharmacy and Pharmacology. Vol. 2, Pg. 764, 1950. | |
| rabbit | LD50 | oral | 3650mg/kg (3650mg/kg) | Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 10, Pg. 289, 1951. |
Related Products
- 4-Aminosalicylic acid
- 6549-60-6
- 65497-07-6
- 65497-29-2
- 654-99-9
- 65501-24-8
- 65505-16-0
- 65505-17-1
- 65505-18-2
- 65505-25-1
- 65505-29-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View