-
Name
4-Ethenylphenol acetate
- EINECS 434-600-2
- CAS No. 2628-16-2
- Article Data46
- CAS DataBase
- Density 1.059 g/cm3
- Solubility
- Melting Point 7-8 °C(lit.)
- Formula C10H10O2
- Boiling Point 260 °C at 760 mmHg
- Molecular Weight 162.188
- Flash Point 94 °C
- Transport Information UN 2810
- Appearance clear colorless liquid
- Safety 36/37
- Risk Codes 22-38-43
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Phenol,4-ethenyl-, acetate (9CI);Phenol, p-vinyl-, acetate (6CI,7CI,8CI);4-Acetoxystyrene;4-Vinylphenyl acetate;p-Acetoxystyrene;
- PSA 26.30000
- LogP 2.25490
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: p-Coumaric Acid With bis(1-oxyl-2,2,6,6-tetramethylpiperidin-4-yl)sebacate; potassium acetate In DMF (N,N-dimethyl-formamide) at 150℃; for 2h; Stage #2: acetic anhydride In DMF (N,N-dimethyl-formamide) at 140℃; for 0.75h; Product distribution / selectivity; | 97.5% |
| Stage #1: p-Coumaric Acid; potassium acetate In ISOPROPYLAMIDE at 135℃; for 3h; Stage #2: acetic anhydride In ISOPROPYLAMIDE at 20℃; for 0.633333h; Product distribution / selectivity; | 71% |
-
-
360068-17-3
4-(1,2-dibromoethyl)phenyl acetate

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| With gallium; bis(cyclopentadienyl)titanium dichloride In tetrahydrofuran at 20℃; for 0.166667h; Inert atmosphere; Ultrasonic irradiation; chemoselective reaction; | 96% |
| With bismuth(III) chloride; gallium In tetrahydrofuran at 20℃; for 0.5h; | 96% |
| With bismuth(III) chloride; indium In methanol at 20℃; for 0.5h; Sonication; chemoselective reaction; | 94% |

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| With molybdenum hexacarbonyl In toluene for 2h; Reflux; chemoselective reaction; | 92% |
-
-
53744-50-6
4'-acetoxyphenylmethylcarbinol

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| With 10H-phenothiazine; 1-ethyl-3-methylimidazolium hydrogensulfate at 150℃; for 6h; Temperature; Reagent/catalyst; | 90% |
| With 4-tert-Butylcatechol; potassium hydroxide In N,N-dimethyl acetamide at 100 - 110℃; Reagent/catalyst; Temperature; | 79.3% |
| With tert-butylcatechol In toluene at 90℃; for 0.5h; Reagent/catalyst; Temperature; Molecular sieve; | 78% |
| With potassium hydrogensulfate at 175 - 200℃; | |
| With acetic anhydride In N,N-dimethyl-formamide at 50℃; |

| Conditions | Yield |
|---|---|
| With 10H-phenothiazine; triethylamine In tert-butyl methyl ether at -5 - 20℃; for 1h; Reagent/catalyst; Solvent; Green chemistry; | 87.7% |
| With triethylamine In dichloromethane at 0℃; for 3h; | 69% |
| With triethylamine In dichloromethane at 20℃; |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide for 0.5h; Heating / reflux; | 86% |
| With 10H-phenothiazine; triethylamine In tert-butyl methyl ether at -5 - 20℃; for 1h; Green chemistry; | 58.6% |
| With dmap; triethylamine In dichloromethane at 23℃; for 0.5h; Inert atmosphere; | 24% |
| With dmap; triethylamine In dichloromethane at 23℃; for 0.5h; Inert atmosphere; | 24% |
| at 80℃; under 760.051 Torr; for 2h; | 80.20 g |

| Conditions | Yield |
|---|---|
| With manganese; trifluoroacetic acid; [2,2]bipyridinyl; cobalt(II) bromide In pyridine; DMF (N,N-dimethyl-formamide) at 50℃; | 81% |

| Conditions | Yield |
|---|---|
| Stage #1: para-coumaric acid With 4-methoxy-phenol In water; N,N-dimethyl-formamide at 136℃; for 6h; Stage #2: acetic anhydride With pyridine In water; N,N-dimethyl-formamide at 30℃; for 0.5h; | 75% |

| Conditions | Yield |
|---|---|
| With Methyltriphenylphosphonium bromide; potassium carbonate In tetrahydrofuran for 6h; Inert atmosphere; Reflux; | 71% |
| Multi-step reaction with 2 steps 1: activated Zn powder / dimethylformamide / 50 °C 2: Ac2O / dimethylformamide / 50 °C View Scheme |
-
-
878-00-2
4-formylphenyl acetate

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 70℃; for 24h; Temperature; Wittig Olefination; | 60.91% |
| With potassium carbonate In 1,4-dioxane for 16h; Reflux; | |
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran at 0℃; for 0.25h; Inert atmosphere; Stage #2: 4-formylphenyl acetate In tetrahydrofuran at 0 - 20℃; for 10h; Inert atmosphere; |
-
-
878-00-2
4-formylphenyl acetate

-
-
2065-66-9
methyl-triphenylphosphonium iodide

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran Inert atmosphere; Reflux; | 44% |
| Stage #1: methyl-triphenylphosphonium iodide With potassium tert-butylate In diethyl ether at 0℃; for 4h; Stage #2: 4-formylphenyl acetate In diethyl ether at 20℃; for 10h; |
-
-
878-00-2
4-formylphenyl acetate

-
-
2065-66-9
methyl-triphenylphosphonium iodide

-
A
-
2628-16-2
p-acetoxystyrene

-
B
-
791-28-6
Triphenylphosphine oxide

| Conditions | Yield |
|---|---|
| With Carbowax 6000 (liquid); potassium carbonate | A 10% B n/a |

-
-
68735-72-8
1-acetoxy-4-(1-acetoxy-ethyl)-benzene

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| at 480℃; | |
| at 480℃; |
-
-
27542-85-4
4-acetoxycinnamic acid

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| With quinoline; copper |

| Conditions | Yield |
|---|---|
| With aluminum oxide; acetic acid at 350℃; under 25 Torr; |

| Conditions | Yield |
|---|---|
| With pyridine; tetrakis(triphenylphosphine) palladium(0); 2,6-di-tert-butyl-4-methyl-phenol; pyridine hydrogenfluoride 1.) toluene, reflux, 8 h, 2.) toluene, RT, 16 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| at 175 - 200℃; |
-
-
948888-24-2
3-bromo-2-(p-hydroxyphenyl) propionic acid

-
-
108-24-7
acetic anhydride

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromo-2-(p-hydroxyphenyl) propionic acid; 2-bromo-3-(p-hydroxyphenyl)propanoic acid With potassium carbonate In N,N-dimethyl-formamide at 65℃; for 1h; Heating / reflux; Stage #2: acetic anhydride In N,N-dimethyl-formamide at 65℃; for 0.25h; Heating / reflux; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium acetate 2: oxygen; chromium oxide-cobalt hydroxide(?)-calcium carbonate catalyst / 145 °C 3: copper oxide-chromium oxide / 130 °C / 102971 Torr / Hydrogenation 4: KHSO4 / 175 - 200 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: oxygen; chromium oxide-cobalt hydroxide(?)-calcium carbonate catalyst / 145 °C 2: copper oxide-chromium oxide / 130 °C / 102971 Torr / Hydrogenation 3: KHSO4 / 175 - 200 °C View Scheme |
-
-
13031-43-1
4-acetyloxyacetophenone

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: palladium/charcoal; methanol / 30 °C / 5884.06 Torr / Hydrogenation 2: acetic acid; Al2O3 / 350 °C / 25 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: copper oxide-chromium oxide / 130 °C / 102971 Torr / Hydrogenation 2: KHSO4 / 175 - 200 °C View Scheme |
-
-
93453-79-3
1-(4-hydroxyphenyl)ethanol

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine; diethyl ether 2: 480 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aqueous NaOH 2: palladium/charcoal; methanol / 30 °C / 5884.06 Torr / Hydrogenation 3: acetic acid; Al2O3 / 350 °C / 25 Torr View Scheme | |
| Multi-step reaction with 3 steps 1: methanol; Raney nickel / 70 - 80 °C / 102971 Torr / Hydrogenation 2: pyridine; diethyl ether 3: 480 °C View Scheme | |
| Multi-step reaction with 3 steps 1: sodium carbonate / ethyl acetate / 8 h / 20 - 30 °C / Large scale 2: hydrogen; 5%-palladium/activated carbon / toluene / 15 - 20 °C / 6000.6 Torr / Large scale 3: potassium hydroxide; 4-tert-Butylcatechol / N,N-dimethyl acetamide / 100 - 110 °C View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 3 h / 0 - 30 °C 2: palladium on activated charcoal; hydrogen / methanol / 5 h / 30 °C / 15001.5 Torr 3: 10H-phenothiazine; 1-ethyl-3-methylimidazolium hydrogensulfate / 6 h / 150 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: BF3 2: aqueous NaOH 3: palladium/charcoal; methanol / 30 °C / 5884.06 Torr / Hydrogenation 4: acetic acid; Al2O3 / 350 °C / 25 Torr View Scheme |

| Conditions | Yield |
|---|---|
| With manganese; trifluoroacetic acid; [2,2]bipyridinyl; cobalt(II) bromide In pyridine; DMF (N,N-dimethyl-formamide) at 50℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 2: potassium carbonate; Methyltriphenylphosphonium bromide / tetrahydrofuran / 6 h / Inert atmosphere; Reflux View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium hydride / dimethyl sulfoxide / 0.25 h / Inert atmosphere; Cooling with ice 1.2: 5 h / 23 °C / Inert atmosphere 2.1: triethylamine; dmap / dichloromethane / 0.5 h / 23 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium hydride / dimethyl sulfoxide / 0.25 h / Inert atmosphere 1.2: 5 h / 23 °C 2.1: triethylamine; dmap / dichloromethane / 0.5 h / 23 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine; glycine / 8 h / 140 °C 2: 2 h / 80 °C / 760.05 Torr View Scheme |
-
-
86952-89-8
4-(oxiran-2-yl)phenyl acetate

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium thioacyanate 2: molybdenum hexacarbonyl / toluene / 2 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| dmap at 85℃; for 60h; | 100% |
-
-
2628-16-2
p-acetoxystyrene

-
-
75-86-5
2-hydroxy-2-methylpropanenitrile

-
-
1132795-53-9
2-(4-acetoxyphenyl)propanenitrile

| Conditions | Yield |
|---|---|
| With 3-[bis-(4-methoxy-phenyl)-phosphanyl]-2H-isoquinolin-1-one; N-{6-[bis-(4-methoxy-phenyl)-phosphanyl]-pyridin-2-yl}-2,2-dimethyl-propionamide; bis(1,5-cyclooctadiene)nickel (0) In toluene at 35℃; for 40h; Inert atmosphere; regioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 40℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 40℃; for 1h; | 100% |
-
-
60556-87-8
2-carbonyl-3-butenenitrile

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| In acetonitrile at 81℃; for 24h; hetero-Diels-Alder cycloaddition; | 99% |

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butylphenoxy(difluoro)borane In 1,4-dioxane at 100℃; for 12h; Prins reaction; | 99% |
| With bismuth(lll) trifluoromethanesulfonate In acetonitrile for 10h; Prins reaction; Heating; | 77% |
-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| With triethylsilane; tetrakis(triphenylphosphine) palladium(0); Selectfluor In acetonitrile at 0℃; for 2h; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen at 20℃; for 18h; Sealed tube; | 99% |
| With 1% Pd on activated carbon; hydrogen In water at 20℃; under 760.051 Torr; for 2h; Reagent/catalyst; Green chemistry; chemoselective reaction; | 99% |
| With hydrogen In toluene at 40℃; under 2250.23 Torr; Catalytic behavior; Reagent/catalyst; Schlenk technique; Glovebox; | |
| With ammonia borane In water at 24.84℃; for 4h; Sonication; Green chemistry; |
-
-
2628-16-2
p-acetoxystyrene

-
-
47241-75-8
(E)-4,4’-diacetoxystilbene

| Conditions | Yield |
|---|---|
| RuCl2(=CHPh)[1,3-ImH2]P(Cy)3 In dichloromethane at 40℃; for 2h; | 98% |
| With Hoveyda-Grubbs catalyst second generation In dichloromethane at 40℃; for 15h; | 93% |
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In methanol; dichloromethane; water at 45℃; for 5h; Ionic liquid; Inert atmosphere; Catalyst solution in CH2Cl2/BMIM incapsulated within polydimethylsiloxane thimble; | 80% |
| (1,3-dimesitylimidazolin-2-ylidene)(C2H4)RuCl2; (p-cymene)RuCl2 In toluene at 85℃; for 2h; | 100 % Turnov. |
-
-
2628-16-2
p-acetoxystyrene

-
-
1417820-87-1
3,3,3-trifluoro-1-(4-acetoxyphenyl)-1-propanol

| Conditions | Yield |
|---|---|
| With tris[2-phenylpyridinato-C2,N]iridium(III); water In acetone at 20℃; for 3h; Schlenk technique; Inert atmosphere; Irradiation; regioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; regioselective reaction; | 98% |
-
-
908133-83-5
ethyl 4-[7-bromo-1-(2-ethoxy-2-oxoethyl)-1H-indol-3-yl]butanoate

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| With dicyclohexylmethylamine; triethylamine In 1,4-dioxane; hexane at 100℃; for 3h; Heck Reaction; Inert atmosphere; | 98% |
-
-
2628-16-2
p-acetoxystyrene

-
-
439584-65-3
4-(1,2-dihydroxyethyl)phenyl acetate

| Conditions | Yield |
|---|---|
| With osmium(VIII) oxide; 4-methylmorpholine N-oxide In water; acetone at 0 - 20℃; for 4h; Inert atmosphere; | 97% |
| Stage #1: p-acetoxystyrene With sodium periodate; acetic acid; lithium bromide at 95℃; for 18h; Prevost-Woodward reaction; Stage #2: With potassium carbonate In methanol at 25℃; for 24h; | |
| With Quinuclidine; osmium(VIII) oxide; tert-butyl alcohol In acetone at 25℃; for 12h; |

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate In water; N,N-dimethyl-formamide | 97% |

-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; potassium acetate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene at 80℃; for 16h; Heck Reaction; Schlenk technique; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite In dichloromethane; water | 97% |
-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane for 24h; Schlenk technique; Inert atmosphere; Irradiation; stereoselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With ethanol; sodium hydroxide at 20℃; for 4h; Inert atmosphere; | 96% |
| With sodium hydroxide In tetrahydrofuran; water at 20℃; for 4h; Cooling with ice; Inert atmosphere; Schlenk technique; | 96% |
| With sodium hydroxide In ethanol at 20℃; for 4h; Inert atmosphere; | 96% |
-
-
2628-16-2
p-acetoxystyrene

-
-
86952-89-8
4-(oxiran-2-yl)phenyl acetate

| Conditions | Yield |
|---|---|
| Stage #1: p-acetoxystyrene With tris(μ-oxo)di[(1,4,7-trimethyl-1,4,7-triazanonane)manganese(IV)] hexafluorophosphate; scandium tris(trifluoromethanesulfonate) In acetonitrile at 20℃; for 0.166667h; Sealed tube; Stage #2: With dihydrogen peroxide In water; acetonitrile for 0.05h; Sealed tube; | 96% |
| With oxone(R); PEG-supported α,α,α-trifluoroacetophenone; sodium hydrogencarbonate In 1,4-dioxane; water at 20℃; for 0.0833333h; | 84% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 - 20℃; for 24h; | 75% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water; acetone at 20℃; for 124h; | 96% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; bathophenanthroline; sodium carbonate In dichloromethane at 100℃; for 60h; Sealed tube; Inert atmosphere; | 96% |
-
-
2628-16-2
p-acetoxystyrene

-
-
13031-43-1
4-acetyloxyacetophenone

| Conditions | Yield |
|---|---|
| With perchloric acid; oxygen; palladium diacetate; p-benzoquinone; sodium nitrite In methanol; water at 20℃; for 5h; Wacker Oxidation; Schlenk technique; Sealed tube; Green chemistry; | 95% |
| With iron(II) chloride In ethanol at 80℃; for 4h; | 88% |
| With tert.-butylhydroperoxide; palladium(II) bis(diketonate) In benzene at 56℃; | 84% |
-
-
591-50-4
iodobenzene

-
-
2628-16-2
p-acetoxystyrene

-
-
93022-30-1, 13041-73-1
Trans-acetic acid 4-styrylphenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine; poly{bis[(N-iPr-acrylamide)5-co-((4-vinylphenyl)PPh2)]PdCl2} In toluene at 100℃; for 20h; Heck reaction; | 95% |
-
-
2628-16-2
p-acetoxystyrene

-
-
880354-47-2
(E)-1-(4'-acetoxyphenyl)-2-(3,4-dimethoxyphenyl)ethene

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium acetate In benzonitrile at 25℃; Heck arylation reaction; | 95% |
-
-
2628-16-2
p-acetoxystyrene

-
-
134029-69-9
(E)-1-(4-acetoxyphenyl)-2-(2,3,4-trimethoxyphenyl)ethene

| Conditions | Yield |
|---|---|
| With Pd2(dba)4; sodium acetate In benzonitrile at 25℃; for 1h; Heck arylation reaction; | 95% |
-
-
2628-16-2
p-acetoxystyrene

-
-
72470-95-2
3,5-dimethoxybenzenediazonium tetrafluoroborate

-
-
18259-14-8, 63366-83-6
(E)-1-(4'-acetoxyphenyl)-2-(3,5-dimethoxyphenyl)ethene

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium acetate In benzonitrile at 25℃; for 3h; Heck arylation reaction; | 95% |
-
-
2628-16-2
p-acetoxystyrene

| Conditions | Yield |
|---|---|
| With sodium acetate; palladium diacetate In methanol at 20℃; for 12h; Heck Reaction; Inert atmosphere; | 95% |
-
-
2628-16-2
p-acetoxystyrene

-
-
1473394-59-0
4-ethoxy-4H-1,4-benzoxaphosphorin-4-oxide

-
-
1473394-82-9
(E)-3-[2-(4-acetoxyphenyl)ethenyl]-4-ethoxy-4H-1,4-benzoxaphosphorin-4-oxide

| Conditions | Yield |
|---|---|
| With copper diacetate; palladium diacetate; trimethylpyruvic acid; silver carbonate at 80℃; for 6h; | 95% |
4-Ethenylphenol acetate Specification
The CAS registry number of 4-Ethenylphenol acetate is 2628-16-2. In addition, the molecular formula is C10H10O2 and the molecular weight is 162.19. It is also called phenol, 4-ethenyl-,1-acetate. What's more, it is a kind of clear colorless liquid and should be stored in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 2.14; (2)ACD/LogD (pH 5.5): 2.14; (3)ACD/LogD (pH 7.4): 2.14; (4)ACD/BCF (pH 5.5): 24.77; (5)ACD/BCF (pH 7.4): 24.77; (6)ACD/KOC (pH 5.5): 346.22; (7)ACD/KOC (pH 7.4): 346.22; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 26.3 Å2; (11)Index of Refraction: 1.546; (12)Molar Refractivity: 48.51 cm3; (13)Molar Volume: 153 cm3; (14)Polarizability: 19.23 ×10-24cm3; (15)Surface Tension: 35.6 dyne/cm; (16)Density: 1.059 g/cm3; (17)Flash Point: 94 °C; (18)Enthalpy of Vaporization: 49.77 kJ/mol; (19)Boiling Point: 260 °C at 760 mmHg; (20)Vapour Pressure: 0.0125 mmHg at 25°C.
Uses of 4-Ethenylphenol acetate: it can react with thiocyanic acid; ammonium salt to get acetic acid 4-thiocyanatoacetyl-phenyl ester. This reaction will need reagents CAN and O2 and solvent methanol. The reaction time is 30 minutes at reaction temperature of 0 °C. The yield is about 68%.
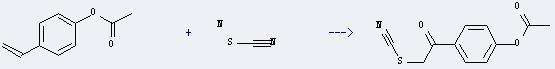
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to the skin and may cause sensitization by skin contact. And it is harmful if swallowed. When you are using it, wear suitable protective clothing and gloves.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(Oc1ccc(cc1)\C=C)C
(2)InChI: InChI=1/C10H10O2/c1-3-9-4-6-10(7-5-9)12-8(2)11/h3-7H,1H2,2H3
(3)InChIKey: JAMNSIXSLVPNLC-UHFFFAOYAV
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LDLo | skin | 200mg/kg (200mg/kg) | United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-1190-1082, | |
| rat | LD50 | oral | 1503mg/kg (1503mg/kg) | United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-1190-1082, | |
| rat | LD50 | skin | > 2gm/kg (2000mg/kg) | United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-1190-1082, |
Related Products
- 4-Ethenylphenol acetate
- 2628-17-3
- 262849-64-9
- 262849-65-0
- 262849-66-1
- 262856-01-9
- 26286-54-4
- 26286-55-5
- 26287-62-7
- 26287-69-4
- 26289-22-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View