-
Name
4-Nitrobenzyl chloroformate
- EINECS 224-708-6
- CAS No. 4457-32-3
- Article Data11
- CAS DataBase
- Density 1.447 g/cm3
- Solubility
- Melting Point 32 °C
- Formula C8H6ClNO4
- Boiling Point 330.8 °C at 760 mmHg
- Molecular Weight 215.593
- Flash Point 153.8 °C
- Transport Information UN 3261 8/PG 2
- Appearance off-white to pale yellow powder
- Safety 26-36/37/39-45-38-28A-27
- Risk Codes 34-37-23/24/25-36/37
-
Molecular Structure
-
Hazard Symbols
 C,
C,  T
T
- Synonyms Formicacid, chloro-, p-nitrobenzyl ester (7CI,8CI);4-Nitrobenzyloxycarbonyl chloride;p-Nitrobenzyl chloroformate;p-Nitrobenzyloxycarbonyl chloride;p-Nitrocarbobenzoxy chloride;
- PSA 72.12000
- LogP 2.99340
Synthetic route

| Conditions | Yield |
|---|---|
| In dichloromethane at 20 - 25℃; for 50h; | 98% |
| With chloroform at 60 - 65℃; im geschlossenen Rohr; | |
| With 1,4-dioxane |
-
-
619-73-8
4-nitrobenzyl chloride

-
-
503-38-8
trichloromethyl chloroformate

-
-
4457-32-3
4-nitrobenzyl chloroformate

| Conditions | Yield |
|---|---|
| With dmap In toluene at 20℃; for 24h; |
-
-
609-36-9
rac-Pro-OH

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
347386-12-3
1-(4-nitrobenzyloxycarbonyl)pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane; water at 0 - 20℃; Shotten-Baumann reaction; | 100% |
-
-
68282-55-3
2-benzyl-1H-imidazole-4-carbaldehyde

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
623906-05-8
2-benzyl-4-formylimidazole-1-carboxylic acid 4-nitrobenzyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane; water at 0℃; for 2.5h; | 100% |
| With sodium bicarbonate In 1,4-dioxane; water | 100% |
| With sodium hydrogencarbonate In 1,4-dioxane; water at 0℃; for 2.5h; | 100% |
-
-
5625-67-2
2-Ketopiperazine

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
623564-23-8
4-p-nitrobenzyloxycarbonyl-2-ketopiperazine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane; dichloromethane at 0℃; for 0.5h; | 100% |
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane; dichloromethane at 0℃; for 0.5h; | 100% |
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane; dichloromethane at 0℃; for 0.5h; | 100% |
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane; dichloromethane; water |
-
-
1322746-31-5
(S)-2-tert-butyloxycarbonylamino-4-(piperidin-4-yl)butanoic acid methyl ester

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
1322746-32-6
(S)-2-tert-butyloxycarbonylamino-4-(1-(4-nitrobenzyloxy)carbonylpiperidin-4-yl)butanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 1.5h; pH=8 - 9; | 100% |

-
-
4457-32-3
4-nitrobenzyl chloroformate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran for 12h; Inert atmosphere; | 100% |

-
-
4457-32-3
4-nitrobenzyl chloroformate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran for 12h; Inert atmosphere; | 100% |

-
-
4457-32-3
4-nitrobenzyl chloroformate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran for 12h; Inert atmosphere; | 100% |

-
-
4457-32-3
4-nitrobenzyl chloroformate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran for 12h; Inert atmosphere; | 100% |
-
-
51-35-4
4R-4-hydroxyproline

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
96034-57-0
(2S,4R)-4-Hydroxy-1-(4-nitrobenzyloxycarbonyl)pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane at 0℃; for 2h; Acylation; | 98% |
| Stage #1: 4R-4-hydroxyproline; 4-nitrobenzyl chloroformate With sodium hydroxide In dichloromethane; water at 0 - 5℃; for 1h; Stage #2: With sulfuric acid In water at 0 - 5℃; | 93% |
| Stage #1: 4R-4-hydroxyproline; 4-nitrobenzyl chloroformate With sodium hydroxide In dichloromethane; water at 0 - 5℃; for 1h; Stage #2: With sulfuric acid In water at 0 - 5℃; | 93% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethyl acetate at 0℃; for 0.25h; | 98% |
-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
2488-14-4, 149263-87-6
N(α)-tert-butoxycarbonyl-histidine methyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform at 0 - 20℃; for 2.5h; | 98% |
| With triethylamine In chloroform at 0 - 20℃; for 2.5h; | 98% |
-
-
18370-81-5
(3-bromopropyl)amine

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
78358-91-5
(3-bromopropyl)-carbamic acid 4-nitrobenzyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran at 20℃; for 2h; | 98% |

-
-
4457-32-3
4-nitrobenzyl chloroformate

| Conditions | Yield |
|---|---|
| Stage #1: (±)-2-endo-amino-3-exo-isopropylbicyclo[2.2.1]heptane hydrochloride With sodium carbonate In dichloromethane; water at 0 - 20℃; for 0.5h; Stage #2: 4-nitrobenzyl chloroformate In dichloromethane; water at 0 - 20℃; for 16h; Inert atmosphere; | 98% |
-
-
85760-58-3
(pivaloyloxy)methyl 6α-<1(R)-hydroxyethyl>penicillanate

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
124093-83-0
(pivaloyloxy)methyl 6α-<1(R)-<<<(p-nitrobenzyl)oxy>carbonyl>oxy>ethyl>penicillanate

| Conditions | Yield |
|---|---|
| With dmap; N-ethyl-N,N-diisopropylamine In dichloromethane for 16h; Ambient temperature; | 97% |
-
-
93591-35-6
(3S,4R)-4-diethoxyphosphinyl-3-<(R)-1-hydroxyethyl>-1-(4-methoxyphenyl)-2-azetidinone

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
93591-37-8
(3S,4R)-4-diethoxyphosphinyl-1-(4-methoxyphenyl)-3-<(R)-1-(4-nitrobenzyloxycarbonyloxy)ethyl>-2-azetidinone

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane for 2h; Ambient temperature; | 97% |
| With dmap In dichloromethane | 79% |
-
-
120476-55-3
N-propyl-6-hydroxyoctylamine

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
120476-71-3
(6-Hydroxy-octyl)-propyl-carbamic acid 4-nitro-benzyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide In diethyl ether Ambient temperature; | 97% |
-
-
39994-75-7
L-threonine methyl ester hydrochloride

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
219851-78-2
Z(NO2)-Thr-OMe

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 3.5h; Acylation; | 97% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane; water at 0 - 20℃; Shotten-Baumann reaction; | 97% |
-
-
648440-61-3
1,12-bis(imidazolin-2-yl)dodecane

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
648440-67-9
1,12-bis[N,N'-(4-nitrobenzyloxycarbonyl)imidazolin-2-yl]dodecane

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform at 0 - 20℃; | 96% |
-
-
21715-90-2
endo-N-hydroxy-5-norbornene-2,3-dicarboxyimide

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
1154758-31-2
N-(4-nitrobenzyl carbonate)bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic acid imide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 4h; | 96% |
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 6h; | 95.1% |
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 2h; | 87.9% |

-
-
4457-32-3
4-nitrobenzyl chloroformate

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 4h; | 96% |
-
-
1195164-50-1
N-hydroxy-5-norbomene-2,3-dicarboxylic acid imide

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
1154758-31-2
N-(4-nitrobenzyl carbonate)bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic acid imide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 4h; | 96% |

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
1374893-41-0
C23H26N2O7S

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 16h; Inert atmosphere; | 96% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane Inert atmosphere; |
-
-
84590-35-2
[3-(2-tert-Butoxycarbonylamino-3-hydroxy-propyl)-1H-indol-4-ylamino]-acetic acid ethyl ester

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
84590-36-3
[[3-(2-tert-Butoxycarbonylamino-3-hydroxy-propyl)-1H-indol-4-yl]-(4-nitro-benzyloxycarbonyl)-amino]-acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In diethyl ether; water for 3h; Ambient temperature; | 95% |
-
-
287119-91-9
methyl 2-methoxy-5-(N-methylamino)phenylacetate

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
287119-95-3
methyl 2-methoxy-5-[N-methyl-N-(4-nitrobenzyloxycarbonyl)]aminophenylacetate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; Acylation; | 95% |

-
-
4457-32-3
4-nitrobenzyl chloroformate

| Conditions | Yield |
|---|---|
| With dmap In chloroform at 20℃; for 0.75h; | 95% |
| With dmap In dichloromethane | 94% |
-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
496048-68-1
(2S,4R)-2-(4-aminomethylthiazol-2-yl)-4-(tert-butyldimethylsilanyloxy)-1-(p-nitrobenzyloxycarbonyl)pyrrolidine

-
-
496048-70-5
(2S,4R)-4-(tert-butyldimethylsilanyloxy)-2-[4-(p-nitrobenzyloxycarbonylaminomethylthiazol-2-yl)]-1-(p-nitrobenzyloxycarbonyl)pyrrolidine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 1h; | 94.2% |
-
-
88192-19-2
3-azidopropylamine

-
-
4457-32-3
4-nitrobenzyl chloroformate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In tetrahydrofuran at 20℃; for 24h; | 94.2% |
-
-
85994-38-3
(3S,4R)-1-(tert-Butyl-dimethyl-silanyl)-4-[2-(tert-butyl-dimethyl-silanyloxy)-ethyl]-3-((R)-1-hydroxy-ethyl)-azetidin-2-one

-
-
4457-32-3
4-nitrobenzyl chloroformate

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane | 94% |
4-Nitrobenzyl chloroformate Specification
The IUPAC name of 4-Nitrobenzyl chloroformate is (4-nitrophenyl)methyl carbonochloridate. With the CAS registry number 4457-32-3 and EINECS 224-708-6, it is also named as Carbonochloridic acid, (4-nitrophenyl)methyl ester. The product's categories are Benzene Derivatives; Active Esters / Additives; Coupling; Peptide Synthesis. It is off-white to pale yellow powder which is stable, but moisture-sensitive. Additionally, this chemical should be sealed in the container, avoided direct sunshine and stored in the cool and dry place.
The other characteristics of this product can be summarized as: (1)ACD/LogP: 2.42; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.42; (4)ACD/LogD (pH 7.4): 2.42; (5)ACD/BCF (pH 5.5): 40.35; (6)ACD/BCF (pH 7.4): 40.35; (7)ACD/KOC (pH 5.5): 490.96; (8)ACD/KOC (pH 7.4): 490.96; (9)#H bond acceptors: 5; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 72.12 Å2; (13)Index of Refraction: 1.571; (14)Molar Refractivity: 48.97 cm3; (15)Molar Volume: 148.9 cm3; (16)Surface Tension: 53.3 dyne/cm; (17)Enthalpy of Vaporization: 57.34 kJ/mol; (18)Vapour Pressure: 0.000163 mmHg at 25°C; (19)Rotatable Bond Count: 3; (20)Exact Mass: 214.998535; (21)MonoIsotopic Mass: 214.998535; (22)Topological Polar Surface Area: 72.1; (23)Heavy Atom Count: 14; (24)Complexity: 220.
Preparation of 4-Nitrobenzyl chloroformate: It can be obtained by carbonyl dichloride and (4-nitro-phenyl)-methanol. This reaction needs solvent CH2Cl2 at temperature of 20-25 °C. The reaction time is 50 hours. The yield is 98%.
.gif)
Uses of 4-Nitrobenzyl chloroformate: It can react with trans-4-hydroxy-L-proline to get trans-4-hydroxy-1-(4-nitro-benzyloxycarbonyl)-L-proline. This reaction needs reagent 4 M aq. NaOH at temperature of 0 °C. The reaction time is 3 hours. The yield is 90%.
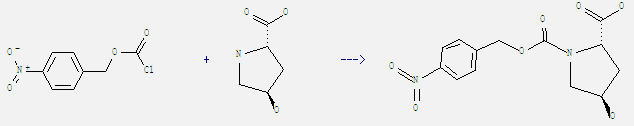
When you are using this chemical, please be cautious about it as the following:
It is not only toxic by inhalation, in contact with skin and if swallowed, but also irritating to eyes and respiratory system. And it can cause burns. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. After using it, take off immediately all contaminated clothing. If you want to contact this product, you must wear suitable protective clothing, gloves and eye/face protection. In case of insufficient ventilation, wear suitable respiratory equipment. Besides, in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
People can use the following data to convert to the molecule structure.
1. SMILES:[O-][N+](=O)c1ccc(cc1)COC(Cl)=O
2. InChI:InChI=1/C8H6ClNO4/c9-8(11)14-5-6-1-3-7(4-2-6)10(12)13/h1-4H,5H2
3. InChIKey:MHSGOABISYIYKP-UHFFFAOYAK
Related Products
- 4-Nitrobenzyl [2R-(2alpha,5beta,6alpha,7beta)]-3-methylene-8-oxo-7-(phenoxyacetamido)-5-thia-1-azabicyclo[4.2.0]octane-2-carboxylate 5-oxide
- 4-Nitrobenzyl 2-diazoacetoacetate
- 4-NITROBENZYL ACETATE
- 4-Nitrobenzyl alcohol
- 4-Nitrobenzyl bromide
- 4-Nitrobenzyl chloride
- 4-Nitrobenzyl chloroformate
- 4-Nitrobenzyl hydrogen malonate
- 4-Nitrobenzylamine
- 4-Nitrobenzylamine hydrochloride
- 4457-67-4
- 4457-71-0
- 4457-72-1
- 4458-15-5
- 4458-18-8
- 445-82-9
- 445-83-0
- 44601-24-3
- 446024-42-6
- 44603-18-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View