-
Name
4-Phenyl-2-pyrrolidinone
- EINECS
- CAS No. 1198-97-6
- Article Data35
- CAS DataBase
- Density 1.108g/cm3
- Solubility
- Melting Point 72.0~78.0℃
- Formula C10H11 N O
- Boiling Point 362.3°Cat760mmHg
- Molecular Weight 161.203
- Flash Point 212.4°C
- Transport Information
- Appearance
- Safety Poison by intraperitoneal route. When heated to decomposition it emits toxic fumes of NOx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 4-Phenyl-2-pyrrolidinone;4-Phenyl-2-pyrrolidone; b-Phenyl-g-aminobutyricacid lactam
- PSA 29.10000
- LogP 1.61890
Synthetic route
-
-
77519-55-2
2-oxo-4-phenylpyrrolidine-3-carboxylic acid

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| In toluene for 5h; Reflux; | 100% |
| at 190℃; for 0.5h; | 58% |
| at 175℃; |
-
-
34687-03-1
4-nitro-3-phenylbutanoic acid methyl ester

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 60℃; under 45004.5 Torr; | 92% |
| With hydrogen; platinum(IV) oxide In methanol for 20h; | 72% |
| With hydrogen; nickel In methanol at 60℃; |

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel dichloride In tetrahydrofuran; methanol at 0 - 20℃; for 1h; | 92% |
-
-
41441-40-1
ethyl 3-phenyl-4-nitrobutanoate

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel(II) chloride hexahydrate In ethanol at 0℃; for 2h; | 88% |
| With sodium tetrahydroborate; nickel(II) chloride hexahydrate In ethanol at 0℃; for 2h; | 82% |
| With hydrogen; palladium on activated charcoal 1.) 2.) toluene, reflux; Yield given. Multistep reaction; | |
| Stage #1: ethyl 3-phenyl-4-nitrobutanoate With hydrogen In ethanol at 20℃; under 1034.32 Torr; Stage #2: In ethanol; toluene Reflux; |

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| Stage #1: C11H11N3O3 In chloroform at 60℃; for 3h; Inert atmosphere; Stage #2: With dmap In chloroform at 20℃; for 2h; Inert atmosphere; | 87% |

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel(II) chloride hexahydrate; water In dichloromethane at -10 - 30℃; for 5h; Concentration; Reagent/catalyst; Temperature; | 85% |
-
-
84872-79-7
4-amino-3-phenylbutanoic acid methyl ester

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol Reflux; | 83% |
-
-
866789-27-7
(+/-)-4-nitro-3-phenylbutyric acid 4'-(4-nitro-3-phenylbutyryloxymethyl)-[2,2']bipyridinyl-4-ylmethyl ester

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With polymer-supported borohydride resin; nickel dichloride In dichloromethane at 20℃; | 60% |

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol at 95℃; for 6h; Sealed tube; | 59% |
-
-
14025-83-3
3-cyano-3-phenylpropanoic acid ethyl ester

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| Stage #1: 3-cyano-3-phenylpropanoic acid ethyl ester With sodium tetrahydroborate; cobalt(II) chloride hexahydrate In tetrahydrofuran; water at 0 - 20℃; for 72h; Stage #2: With ammonia In tetrahydrofuran; water | 57% |
| Multi-step reaction with 2 steps 1: H2, conc. HCl / 10percent Pd/C / methanol / 2068.6 Torr 2: Et3N / toluene / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; sodium chloride at 0 - 180℃; under 750.075 Torr; | 44% |

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; zinc In ethanol Heating; | 36% |
-
-
55790-16-4
(2-nitro-1-phenyl-ethyl)-malonic acid dimethyl ester

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With palladium on activated carbon; hydrogen In o-xylene at 130℃; under 11251.1 Torr; for 18h; Catalytic behavior; | 35% |
| Multi-step reaction with 3 steps 1: hydrogen / methanol / 2.5 h / 20 °C / 3102.97 Torr 2: sodium hydroxide / ethanol / 48 h / 20 °C 3: toluene / 5 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; sodium chloride at 0 - 180℃; under 750.075 Torr; | 32% |
-
-
52450-32-5
2-oxo-4-phenyl-3-ethoxycarbonylpyrrolidine

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol; water | |
| Multi-step reaction with 2 steps 1: ethanolic KOH 2: 175 °C View Scheme | |
| With sodium carbonate for 6h; Reagent/catalyst; Reflux; | 3.3 g |

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In toluene Heating; |

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With diethyl ether; nickel at 70 - 90℃; Hydrogenation; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 96 percent / 1,1,3,3-tetramethylguanidine / 3 h / Heating 2: 72 percent / H2 / PtO2 / methanol / 20 h View Scheme | |
| Multi-step reaction with 2 steps 1: 1,1,3,3-tetramethylguanidine / 70 °C 2: H2 / Raney nickel / methanol / 60 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 1,8-diazabicyclo[5.4.0]undec-7-ene / methanol 2: hydrogen / platinum(IV) oxide / ethanol View Scheme | |
| Multi-step reaction with 3 steps 1: N,N,N',N'-tetramethylguanidine / 42 h / 20 °C 2: palladium 10% on activated carbon; acetic acid; hydrogen / dichloromethane / 20 °C 3: triethylamine / ethanol / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: methanol; H2O / 60 °C 2: H2, conc. HCl / 10percent Pd/C / methanol / 2068.6 Torr 3: Et3N / toluene / Heating View Scheme |
-
-
55790-17-5
3-methoxycarbonyl-4-phenyl-2-pyrrolidone

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 89.6 percent / 10percent aq. KOH / 5 h / Heating 2: 58 percent / 0.5 h / 190 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / ethanol / 48 h / 20 °C 2: toluene / 5 h / Reflux View Scheme |

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: acetic acid / chloroform / 1 h / 0 - 20 °C 1.2: 20 °C 2.1: trifluoroacetic acid / 1,2-dichloro-ethane / 2 h / 80 °C 3.1: nickel dichloride; sodium tetrahydroborate / methanol; tetrahydrofuran / 1 h / 0 - 20 °C View Scheme |

-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trifluoroacetic acid / 1,2-dichloro-ethane / 2 h / 80 °C 2: nickel dichloride; sodium tetrahydroborate / methanol; tetrahydrofuran / 1 h / 0 - 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: cesium fluoride; tetrabutyl-ammonium chloride; bis-triphenylphosphine-palladium(II) chloride / toluene; water / 18 h / 20 °C / Inert atmosphere 2.1: acetic acid / chloroform / 1 h / 0 - 20 °C 2.2: 20 °C 3.1: trifluoroacetic acid / 1,2-dichloro-ethane / 2 h / 80 °C 4.1: nickel dichloride; sodium tetrahydroborate / methanol; tetrahydrofuran / 1 h / 0 - 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / 20 °C 2.1: hydrogen / ethanol / 20 °C / 1034.32 Torr 2.2: Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / methanol; water / 24 h / 20 - 30 °C 2: nickel(II) chloride hexahydrate; water; sodium tetrahydroborate / dichloromethane / 5 h / -10 - 30 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: tetrahydrofuran / 20 °C 2.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / 20 °C 3.1: hydrogen / ethanol / 20 °C / 1034.32 Torr 3.2: Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: aminopropyl-silica-based catalyst; dimethylaminopropyl-silica-based solid / o-xylene / 18 h / 70 °C / Green chemistry 2: palladium on activated carbon; hydrogen / o-xylene / 18 h / 130 °C / 11251.1 Torr View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium tert-butylate / toluene; methanol / 0.17 h / 20 °C / 4500.45 Torr / Inert atmosphere 1.2: 0.11 h / 25 °C / 4500.45 Torr / Inert atmosphere 2.1: tetrabutyl ammonium fluoride / toluene; tetrahydrofuran / 1 h / 50 °C / 6000.6 Torr 3.1: hydrogen; palladium 10% on activated carbon / methanol / 60 °C / 45004.5 Torr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: calcined hydrotalcite / ethanol / 24 h / Reflux 2: nickel(II) chloride hexahydrate; sodium tetrahydroborate / ethanol / 2 h / 0 °C View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / dichloromethane / 24 h / 25 °C 2: calcined hydrotalcite / 24 h / Reflux 3: nickel(II) chloride hexahydrate; sodium tetrahydroborate / ethanol / 2 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; sodium hypochlorite; potassium bromide / water; dichloromethane / 0.21 h / 0 °C 2.1: potassium tert-butylate / toluene; methanol / 0.17 h / 20 °C / 4500.45 Torr / Inert atmosphere 2.2: 0.11 h / 25 °C / 4500.45 Torr / Inert atmosphere 3.1: tetrabutyl ammonium fluoride / toluene; tetrahydrofuran / 1 h / 50 °C / 6000.6 Torr 4.1: hydrogen; palladium 10% on activated carbon / methanol / 60 °C / 45004.5 Torr View Scheme |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
106-96-7
propargyl bromide

-
-
137518-26-4
N-propargyl-4-phenyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetrabutylammomium bromide In tetrahydrofuran at 0 - 20℃; for 6h; | 97% |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
42340-98-7
(R)-1-(1-naphthyl)ethyl isocyanate

-
-
916345-91-0
N-<(R)-1-(1-naphthyl)ethyl>-4-phenyl-2-pyrrolidone-1-carboxamide

| Conditions | Yield |
|---|---|
| In benzene for 24h; Heating; | 95% |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
106869-48-1
4-phenyl-1-(trimethylsilyl)-2-pyrrolidinone

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 0.333333h; Heating; | 90% |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water for 12h; Reflux; | 88% |
| With hydrogenchloride In water for 12h; Reflux; | 88% |
| With hydrogenchloride In water for 16h; Sealed tube; Reflux; | 65% |
| With hydrogenchloride; water In water for 16h; Reflux; | 55% |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
74-88-4
methyl iodide

-
-
54520-84-2
1-methyl-4-phenyl-pyrrolidin-2-one

| Conditions | Yield |
|---|---|
| Stage #1: 4-phenylpyrrolidin-2-one With sodium hydride In tetrahydrofuran; mineral oil for 0.5h; Sealed tube; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran; mineral oil at 20℃; Sealed tube; Inert atmosphere; | 88% |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
96-34-4
methyl chloroacetate

-
-
68497-63-2
methyl-2-oxo-4-phenylpyrrolidine-1-acetate

| Conditions | Yield |
|---|---|
| Stage #1: 4-phenylpyrrolidin-2-one With potassium tert-butylate In N,N-dimethyl-formamide at -10℃; for 2h; Stage #2: methyl chloroacetate In N,N-dimethyl-formamide at -10 - 0℃; for 2h; Concentration; Temperature; Solvent; | 84% |
| With sodium methylate 1.) toluene, 150-160 deg C, 2.) toluene, 60-70 deg C, 2 h; Yield given. Multistep reaction; | |
| In tetrahydrofuran; water; mineral oil |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
924650-17-9
3-bromo-5-[methyl(methylsulfonyl)amino]benzoic acid methyl ester

-
-
929041-94-1
3-[methyl(methylsulfonyl)amino]-5-(2-oxo-4-phenylpyrrolidine-1-yl)benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With caesium carbonate; tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane at 110℃; for 7h; | 82% |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
368-39-8
triethyloxonium fluoroborate

-
-
22349-33-3
Ethoxy-2 phenyl-4 Δ1-pyrroline

| Conditions | Yield |
|---|---|
| 81% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene | 76.5% |
-
-
50-00-0
formaldehyd

-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
68116-87-0
1-chloromethyl-4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane for 1h; Heating; | 67.5% |

| Conditions | Yield |
|---|---|
| With thionyl chloride; triethylamine In toluene | A 58.5% B 65.4% |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; 1,3-bis(methylamino)propane In 1,4-dioxane for 24h; Reflux; | 60% |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
1203050-38-7
4-iodo-N-(quinolin-8-yl)benzamide

-
-
1437789-57-5
C26H21N3O2

| Conditions | Yield |
|---|---|
| With copper(l) iodide; caesium carbonate; N,N`-dimethylethylenediamine In 1,4-dioxane at 140℃; Microwave irradiation; | 59% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 57% |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide; mineral oil at 80℃; for 1h; Inert atmosphere; | 55% |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
100-39-0
benzyl bromide

-
-
108303-98-6
1-benzyl-4-phenylpyrrolidin-2-one

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; sodium hydride In tetrahydrofuran at 0 - 20℃; for 0.5h; | 52% |
| With sodium hydride 1.) xylene, reflux, 5 h, 2.) xylene, reflux, 4 h; Yield given. Multistep reaction; |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
210691-38-6
1-(2,2,2-trifluoroacetyl)indolin-5-ylsulfonyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: 4-phenylpyrrolidin-2-one With n-butyllithium In tetrahydrofuran for 0.5h; Stage #2: 1-(2,2,2-trifluoroacetyl)indolin-5-ylsulfonyl chloride In tetrahydrofuran at 0℃; for 4h; | 45% |
-
-
1198-97-6
4-phenylpyrrolidin-2-one

-
-
22349-31-1
4-phenyl-2-pyrrolidinethione

| Conditions | Yield |
|---|---|
| With diphosphorus pentasulfide In o-xylene Heating; | 40% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-phenylpyrrolidin-2-one With sodium hydride In N,N-dimethyl-formamide at 20 - 50℃; for 1h; Metallation; Stage #2: 2-(diethylamino)ethyl chloride In N,N-dimethyl-formamide at 80℃; for 8h; Alkylation; Stage #3: oxalic acid In diethyl ether; ethanol acid-base reaction; | 37.5% |

4-Phenyl-2-pyrrolidinone Chemical Properties
Empirical Formula of 4-Phenylpyrrolidone-2 (CAS NO.1198-97-6): C10H11NO
Molecular Weight: 161.2004 g/mol
Index of Refraction: 1.55
Density: 1.108 g/cm3
Flash Point: 212.4 °C
Enthalpy of Vaporization: 60.82 kJ/mol
Boiling Point: 362.3 °C at 760 mmHg
Vapour Pressure: 1.96E-05 mmHg at 25 °C
Structure of 4-Phenylpyrrolidone-2 (CAS NO.1198-97-6):
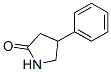
IUPAC Name of 4-Phenylpyrrolidone-2 (CAS NO.1198-97-6): 4-Phenylpyrrolidin-2-one
4-Phenyl-2-pyrrolidinone Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LD50 | unreported | 352mg/kg (352mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: ANALGESIA | Russian Pharmacology and Toxicology Vol. 44, Pg. 22, 1981. |
| mouse | LD50 | intraperitoneal | 325mg/kg (325mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA | Archivum Immunologiae et Therapiae Experimentalis. Vol. 23, Pg. 733, 1975. |
| mouse | LD50 | unreported | 352mg/kg (352mg/kg) | AUTONOMIC NERVOUS SYSTEM: "SMOOTH MUSCLE RELAXANT (MECHANISM UNDEFINED, SPASMOLYTIC)" BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: MUSCLE WEAKNESS | Pharmaceutical Chemistry Journal Vol. 14, Pg. 776, 1980. |
4-Phenyl-2-pyrrolidinone Safety Profile
Poison by intraperitoneal route. When heated to decomposition it emits toxic fumes of NOx.
4-Phenyl-2-pyrrolidinone Specification
4-Phenylpyrrolidone-2 ,its cas register number is 1198-97-6. It also can be called 4-Phenyl-2-pyrrolidinone ; 2-Pyrrolidinone, 4-phenyl- ;and Phenylpyrrolidone .
Related Products
- 4-Phenyl-2-pyrrolidinone
- 1198-98-7
- 119-90-4
- 1199-04-8
- 119904-90-4
- 1199-08-2
- 119911-68-1
- 119914-60-2
- 119-91-5
- 119915-47-8
- 1199-20-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View