-
Name
5-(4-DIMETHYLAMINOBENZYLIDENE)RHODANINE
- EINECS 208-625-2
- CAS No. 536-17-4
- Article Data17
- CAS DataBase
- Density 1.368 g/cm3
- Solubility Insoluble in water, 993.7(mg/L) at 25°C
- Melting Point 285-288 °C(lit.)
- Formula C12H12N2OS2
- Boiling Point 430 °C at 760 mmHg
- Molecular Weight 264.372
- Flash Point 213.8 °C
- Transport Information
- Appearance red fluffy powder
- Safety 22-24/25-36/37-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Rhodanine,5-[p-(dimethylamino)benzylidene]- (6CI,7CI,8CI);4'-(Dimethylamino)benzalrhodanine;5-[4-(Dimethylamino)benzylidene]-2-thioxo-4-thiazolidone;NSC 5042;p-(Dimethylamino)benzal-5-rhodanine;
- PSA 89.73000
- LogP 2.57020
Synthetic route
-
-
141-84-4
2-thioxo-4-thiazolidinone

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

| Conditions | Yield |
|---|---|
| With thiourea; urea at 110℃; for 0.0833333h; Knoevenagel condensation; Neat (no solvent); | 96% |
| With (2-hydroxyethyl)ammonium formate at 20℃; for 0.05h; Knoevenagel condensation; neat (no solvent); | 90% |
| With sodium acetate; acetic acid at 120℃; | 90% |
-
-
141-84-4
2-thioxo-4-thiazolidinone

-
-
889-37-2, 1613-99-6
N,N-dimethyl-4-((phenylimino)methyl)aniline

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

| Conditions | Yield |
|---|---|
| In acetic acid |

| Conditions | Yield |
|---|---|
| With hydrogen cation In water |

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H+ / H2O View Scheme |
-
-
1660-94-2
Tetraethyl methylenediphosphonate

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
634915-49-4
[1-(diethoxy-phosphoryl)-2-(4-dimethylamino-phenyl)-2-(4-oxo-2-thioxo-thiazolidin-5-yl)-ethyl]-phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol; N,N-dimethyl-formamide for 0.133333h; Michael addition reaction; microwave irradiation; | 87% |
-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
13939-06-5, 199620-15-0
molybdenum hexacarbonyl

-
-
952576-76-0
Mo(CO)5[5-(4-dimethylaminobenzylidene)rhodanine]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Irradiation (UV/VIS); Mo complex and rhodanine deriv. dissolved in THF; soln. irradiated for 2h using 400 W medium pressure Hg lamp; solvent evapd. under vac.; solid extd. with CH2Cl2; petroleum ether added; ppt. washed with petroleum ether; dried under vac.; unreacted Cr complex sublimed out in vac.; elem. anal.; | 82% |
-
-
50-00-0
formaldehyd

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
51207-85-3
4-amino-N,N-diethylbenzamide

-
-
104183-56-4
5-(p-Dimethylaminobenzylidene)-3--4-oxo-thiazolidine-2-thione

| Conditions | Yield |
|---|---|
| In ethanol; water Heating; | 81% |

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
22303-36-2
N-(4-methoxyphenyl)-2-chloroacetamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; sodium iodide In N,N-dimethyl-formamide at 20℃; for 36h; | 80% |
-
-
50-00-0
formaldehyd

-
-
42837-37-6
4-(N-piperidinocarbonyl)aniline

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
104183-54-2
5-(p-Dimethylaminobenzylidene)-3--4-oxo-thiazolidine-2-thione

| Conditions | Yield |
|---|---|
| In ethanol; water Heating; | 78% |

-
-
50-00-0
formaldehyd

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
79834-39-2
p-(4-Phenylpiperazin-1-ylcarbamoyl)aniline

-
-
104183-53-1
5-(p-Dimethylaminobenzylidene)-3--4-oxo-thiazolidine-2-thione

| Conditions | Yield |
|---|---|
| In ethanol; water Heating; | 74% |

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
199620-14-9, 13007-92-6
chromium(0) hexacarbonyl

-
-
952576-75-9
Cr(CO)5[5-(4-dimethylaminobenzylidene)rhodanine]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Irradiation (UV/VIS); Cr complex and rhodanine deriv. dissolved in THF; soln. irradiated for 2h using 400 W medium pressure Hg lamp; solvent evapd. under vac.; solid extd. with CH2Cl2; petroleum ether added; ppt. washed with petroleum ether; dried under vac.; unreacted Cr complex sublimed out in vac.; elem. anal.; | 73% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; sodium iodide In N,N-dimethyl-formamide at 20℃; for 36h; | 73% |
-
-
50-00-0
formaldehyd

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
51207-86-4
(4-amino-phenyl)-morpholin-4-yl-methanone

-
-
104183-55-3
5-(p-Dimethylaminobenzylidene)-3--4-oxo-thiazolidine-2-thione

| Conditions | Yield |
|---|---|
| In ethanol; water Heating; | 72% |


| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; sodium iodide In N,N-dimethyl-formamide at 20℃; for 36h; | 71% |
-
-
14040-11-0
tungsten hexacarbonyl

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
952576-77-1
W(CO)5[5-(4-dimethylaminobenzylidene)rhodanine]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Irradiation (UV/VIS); W complex and rhodanine deriv. dissolved in THF; soln. irradiated for 2 h using 400 W medium pressure Hg lamp; solvent evapd. under vac.; solid extd. with CH2Cl2; petroleum ether added; ppt. washed with petroleum ether; dried under vac.; unreacted Cr complex sublimed out in vac.; elem. anal.; | 70% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; sodium iodide In N,N-dimethyl-formamide at 20℃; for 36h; | 70% |
-
-
14220-21-4
rhenium(I) pentacarbonyl bromide

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
952576-78-2
cis-Re(CO)4[5-(4-dimethylaminobenzylidene)rhodanine]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Irradiation (UV/VIS); Re complex and rhodanine deriv. dissolved in THF; soln. irradiated for 2h using 400 W medium pressure Hg lamp; solvent evapd. under vac.; solid extd. with CH2Cl2; petroleum ether added; ppt. washed with petroleum ether; dried under vac.; unreacted Cr complex sublimed out in vac.; elem. anal.; | 68% |
-
-
12146-36-0
tetracarbonylnorbornadienchrom

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
1123204-07-8
cis-[(5-(4-dimethylaminobenzylidene)rhodanine)tetra-carbonylchromate(0)]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: norbornadiene; Irradiation (UV/VIS); Ar, equimol., a soln. of metal compd. irradiated for 1.5 h, cooled, N compd. added, stirred for 3 h; solvent evapd. (vac.), extd. (CH2Cl2). pptd. (petroleum ether), ppt. washed (petroleum ether), dried (vac.); elem. anal.; | 62% |
-
-
12146-37-1, 124717-04-0
(bicyclo[2.2.1]hepta-2,5-diene)tetracarbonylmolybdenum(0)

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
1123204-08-9
cis-[(5-(4-dimethylaminobenzylidene)rhodanine)tetra-carbonylmolybdate(0)]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: norbornadiene; Irradiation (UV/VIS); Ar, equimol., a soln. of metal compd. irradiated for 1.5 h, cooled, N compd. added, stirred for 3 h; solvent evapd. (vac.), extd. (CH2Cl2). pptd. (petroleum ether), ppt. washed (petroleum ether), dried (vac.); elem. anal.; | 54% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 20℃; for 72h; | 52% |
-
-
867-13-0
diethoxyphosphoryl-acetic acid ethyl ester

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 20℃; for 72h; | 50% |
-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
682-30-4
Diethyl vinylphosphonate

-
A
-
31982-65-7
5-(4'-dimethylamino-phenylmethylene)-2-(ethylthio)-1,3-thiazol-4-one

| Conditions | Yield |
|---|---|
| With lithium hydride In N,N-dimethyl-formamide for 12h; Heating; | A 28% B 44% |


| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With lithium hydride In N,N-dimethyl-formamide for 1h; Heating; Stage #2: 5-(4-dimethylaminobenzylidene)rhodanine In N,N-dimethyl-formamide Heating; | A 30% B 33% |

-
-
867-13-0
diethoxyphosphoryl-acetic acid ethyl ester

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine



-
C
-
762292-91-1
[7-(4-dimethylamino-phenyl)-3-ethyl-5-oxo-2-thioxo-3,5,6,7-tetrahydro-2H-pyrano[2,3-d]thiazol-6-yl]-phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 24h; Heating; | A 28% B 14% C 30% |

-
-
1067-74-9
Methyl diethylphosphonoacetate

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine



-
C
-
762292-91-1
[7-(4-dimethylamino-phenyl)-3-ethyl-5-oxo-2-thioxo-3,5,6,7-tetrahydro-2H-pyrano[2,3-d]thiazol-6-yl]-phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 24h; Heating; | A 30% B 13% C 29% |

-
-
1660-94-2
Tetraethyl methylenediphosphonate

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine


-
B
-
634915-49-4
[1-(diethoxy-phosphoryl)-2-(4-dimethylamino-phenyl)-2-(4-oxo-2-thioxo-thiazolidin-5-yl)-ethyl]-phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: Tetraethyl methylenediphosphonate With sodium hydride In N,N-dimethyl-formamide at 0℃; for 1h; Stage #2: 5-(4-dimethylaminobenzylidene)rhodanine In N,N-dimethyl-formamide at 20℃; | A 22% B 28% |

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
99981-54-1
3-(4-dimethylamino-phenyl)-propionic acid amide

| Conditions | Yield |
|---|---|
| With methanol; nickel |
-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
857205-41-5
3-(4-dimethylamino-phenyl)-2-thioxo-propionic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
A
-
28996-10-3
5-(4-dimethylamino-benzylidene)-2-methylsulfanyl-thiazol-4-one

-
B
-
23517-84-2
5-(4-dimethylamino-benzylidene)-3-methyl-2-thioxo-thiazolidin-4-one

| Conditions | Yield |
|---|---|
| In ethanol |

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
75-03-6
ethyl iodide

-
-
31982-65-7
5-(4'-dimethylamino-phenylmethylene)-2-(ethylthio)-1,3-thiazol-4-one

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane |
-
-
50-00-0
formaldehyd

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
334-22-5
N,N-bis(chloro-2-ethyl)amine

-
-
7751-32-8
3-{[bis-(2-chloro-ethyl)-amino]-methyl}-5-(4-dimethylamino-benzylidene)-2-thioxo-thiazolidin-4-one

| Conditions | Yield |
|---|---|
| In ethanol at 0 - 5℃; |

-
-
536-17-4
5-(4-dimethylaminobenzylidene)rhodanine

-
-
4303-28-0
5-(4-dimethylamino-benzylidene)-thiazolidine-2,4-dithione

| Conditions | Yield |
|---|---|
| With tetraphosphorus decasulfide In 1,4-dioxane for 0.5h; Heating; |
5-(4-Dimethylaminobenzylidene)rhodanine Chemical Properties
IUPAC Name: (5E)-5-[(4-Dimethylaminophenyl)methylidene]-2-sulfanylidene-1,3-thiazolidin-4-one
CAS: 536-17-4
Formula: C12H12N2OS2
Molecular Weight: 264.37
Molecular Structure of (5E)-5-[(4-Dimethylaminophenyl)methylidene]-2-sulfanylidene-1,3-thiazolidin-4-one (CAS NO.536-17-4):
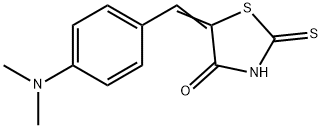
Density: 1.36 g/cm3
Flash Point: 213.8 °C
Melting Point: 285-288 °C(lit.)
Boiling Point: 430 °C at 760 mmHg
Index of Refraction: 1.703
Molar Refractivity: 74.94 cm3
Molar Volume: 193.1 cm3
Polarizability: 29.7×10-24cm3
Surface Tension: 68.3 dyne/cm
Enthalpy of Vaporization: 68.53 kJ/mol
Appearance: red fluffy powder
Vapour Pressure: 1.35E-07 mmHg at 25°C
Water Solubility: 993.7(mg/L) at 25°C
Product Categories: building blocks;heterocyclic building blocks;thiazoles.
5-(4-Dimethylaminobenzylidene)rhodanine Toxicity Data With Reference
| 1. | ipr-mus LD50:150 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD603-561 . |
5-(4-Dimethylaminobenzylidene)rhodanine Consensus Reports
Reported in EPA TSCA Inventory.
5-(4-Dimethylaminobenzylidene)rhodanine Safety Profile
Poison by intraperitoneal route. When heated to decomposition it emits very toxic fumes of NOx and SOx.
Safety Information about (5E)-5-[(4-Dimethylaminophenyl)methylidene]-2-sulfanylidene-1,3-thiazolidin-4-one (CAS NO.536-17-4):
Hazard Codes:
Xi: 
Risk Statements about (5E)-5-[(4-Dimethylaminophenyl)methylidene]-2-sulfanylidene-1,3-thiazolidin-4-one (CAS NO.536-17-4):
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements about (5E)-5-[(4-Dimethylaminophenyl)methylidene]-2-sulfanylidene-1,3-thiazolidin-4-one (CAS NO.536-17-4):
S22: Do not breathe dust.
S23: Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer).
S24/25: Avoid contact with skin and eyes.
S36/37: Wear suitable protective clothing and gloves.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany: 3
RTECS: VI8090000
5-(4-Dimethylaminobenzylidene)rhodanine Specification
The chemical synonyms of (5E)-5-[(4-Dimethylaminophenyl)methylidene]-2-sulfanylidene-1,3-thiazolidin-4-one (CAS NO.536-17-4) are 5-((4-(Dimethylamino)phenyl)methylene)-2-thioxo-4-thiazolidinon ; 5-(p-(Dimethylamino)benzylidene)-rhodanin ; 5-(p-Dimethylaminobenzal)rhodanine ; 5-(p-Dimethylaminobenzoylidene)rhodanine ; 5-[[4-(Dimethylamino)phenyl]methylene]-2-thioxo-4-thiazolidinon ; 5-[4-(Dimethylamino)phenyl]methylene-2-thioxo-4-Thiazolidinone ; p-(Dimethylamino)benzal-5-rhodanine ; p-Dimethylaminobenzalrhodanin . As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Use water spray, dry chemical, carbon dioxide, or chemical foam. Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Store in a cool, dry place. Store in a tightly closed container. It can be used as organic reagents pharmaceutical intermediate.
Related Products
- 5-(4-Dimethylaminobenzylidene)rhodanine
- 53619-11-7
- 53619-53-7
- 53619-77-5
- 53623-37-3
- 53623-42-0
- 536-25-4
- 53629-18-8
- 53629-19-9
- 53631-18-8
- 53632-49-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View