-
Name
5,6-Dimethylbenzimidazole
- EINECS 209-488-1
- CAS No. 582-60-5
- Article Data61
- CAS DataBase
- Density 1.145 g/cm3
- Solubility soluble
- Melting Point 202-205 °C(lit.)
- Formula C9H10N2
- Boiling Point 376.8 °C at 760 mmHg
- Molecular Weight 146.192
- Flash Point 208.6 °C
- Transport Information
- Appearance light beige powder
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Benzimidazole, 5,6-dimethyl-;1H-Benzimidazole, 5,6-dimethyl-;5,6-dimethyl-1H-benzoimidazole;5-23-06-00454 (Beilstein Handbook Reference);5,6-dimethyl-1H-benzimidazole;Dimezol base;Dimedazol;Dimethylbenzimidazole;
- PSA 28.68000
- LogP 2.17970
Synthetic route
-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
122-51-0
orthoformic acid triethyl ester

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With gallium(III) triflate at 20℃; for 0.1h; | 99% |
| With aminosulfonic acid In methanol at 20℃; for 1h; | 94% |
| iodine In acetonitrile at 20℃; for 0.666667h; | 93% |
-
-
67-56-1
methanol

-
-
6972-71-0
4,5-dimethyl-2-nitrobenzenamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With iron; ammonium chloride at 68 - 70℃; for 48h; Inert atmosphere; | 99% |
-
-
4252-31-7
N-formylbenzamide

-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In tetrahydrofuran at 50℃; for 9h; Inert atmosphere; Green chemistry; | 96% |
-
-
64-18-6
formic acid

-
-
610-23-1
4,5-dimethyl-1,2-dinitrobenzene

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With gold nano particles supported on rutile TiO2 In toluene at 70℃; under 750.075 Torr; for 6h; Inert atmosphere; chemoselective reaction; | 95% |
-
-
6972-71-0
4,5-dimethyl-2-nitrobenzenamine

-
-
122-51-0
orthoformic acid triethyl ester

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With 10% Pd/C; hydrogen; acetic acid In methanol at 20℃; under 760.051 Torr; | 94% |
| With iron; ytterbium(III) triflate at 75℃; for 3h; | 82% |
-
-
64-18-6
formic acid

-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With tetrabutyl-ammonium chloride In water; toluene at 160℃; for 0.166667h; Microwave irradiation; | 93% |
| With chloro-trimethyl-silane In water; N,N-dimethyl-formamide at 120℃; for 0.166667h; Microwave irradiation; | 93% |
| In methanol at 20℃; under 1551.49 Torr; Flow reactor; Inert atmosphere; | 38% |
-
-
124-38-9
carbon dioxide

-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With bis[1,2-bis(diphenylphosphine)ethane]ruthenium dichloride; hydrogen at 120℃; under 112511 Torr; for 40h; Green chemistry; | 93% |
| With dimethylamine borane In ethanol; water at 100℃; under 750.075 Torr; for 24h; | 93% |
| With tris(pentafluorophenyl)borate; phenylsilane In tetrahydrofuran at 120℃; under 7500.75 Torr; for 24h; Autoclave; | 62% |
-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With Imidazole hydrochloride at 120℃; for 6h; | 93% |
| With phenylsilane at 120℃; for 12h; | 50% |
| With Triethoxysilane; carbon dioxide; tris(pentafluorophenyl)borate at 120℃; for 24h; | 99 %Spectr. |
-
-
15776-99-5
1-benzyl-5,6-dimethyl-1H-benzo[d]imidazole

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With triethylsilane; palladium 10% on activated carbon In tetrahydrofuran at 20℃; for 18h; Inert atmosphere; | 92% |
-
-
144-62-7
oxalic acid

-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 120℃; for 6h; Green chemistry; | 92% |
-
-
64-18-6
formic acid

-
-
6972-71-0
4,5-dimethyl-2-nitrobenzenamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; triethylamine at 150℃; for 0.0833333h; Microwave irradiation; | 91% |
-
-
67-56-1
methanol

-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In 1,4-dioxane at 130℃; for 48h; Autoclave; | 90% |
-
-
64-18-6
formic acid

-
-
132382-43-5
N-(2-amino-4,5-dimethylphenyl)formamide

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| at 110℃; for 6h; Temperature; | 87.9% |

| Conditions | Yield |
|---|---|
| With rose bengal; water; oxygen; sodium chloride In N,N-dimethyl-formamide at 25℃; for 48h; Irradiation; Green chemistry; | 87% |
-
-
124-38-9
carbon dioxide

-
-
6972-71-0
4,5-dimethyl-2-nitrobenzenamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; phenylsilane; 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine In acetonitrile at 70℃; under 7500.75 Torr; for 15h; | 84% |
| With gold on titanium oxide; hydrogen In 1-methyl-pyrrolidin-2-one at 100℃; under 60006 Torr; for 12h; Sealed tube; Green chemistry; | 81% |
-
-
124-38-9
carbon dioxide

-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
A
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With phenylsilane at 50℃; under 22502.3 Torr; for 3h; Pressure; Autoclave; | A 78% B 11% |

-
-
109-86-4
2-methoxy-ethanol

-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With oxygen; toluene-4-sulfonic acid In water at 110℃; Sealed tube; | 77% |
-
-
2280-44-6
D-Glucose

-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; trifluorormethanesulfonic acid; water at 100℃; for 1h; Sealed tube; | 75% |
-
-
67-56-1
methanol

-
-
6972-71-0
4,5-dimethyl-2-nitrobenzenamine

-
A
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
B
-
1128-27-4
1,5,6-trimethylbenzimidazole

| Conditions | Yield |
|---|---|
| With Cu-Zn-PMO at 250℃; for 3h; Sealed tube; Supercritical conditions; Green chemistry; | A 73% B n/a |

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane In acetonitrile at 70℃; for 24h; | 60% |
-
-
78288-57-0
1-Amino-5,6-dimethylbenzimidazole

-
A
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
B
-
127845-08-3
Bis-(5,6-dimethyl-benzoimidazol-1-yl)-diazene

| Conditions | Yield |
|---|---|
| With bromine; sodium carbonate In water at 0 - 5℃; for 1h; | A 55% B 10% |
-
-
67-68-5
dimethyl sulfoxide

-
-
3171-45-7
4,5-dimethyl-1,2-phenylenediamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With ammonium acetate; water at 140℃; for 10h; Inert atmosphere; Schlenk technique; | 42% |
| With ammonium acetate In water at 140℃; for 11h; Schlenk technique; Inert atmosphere; | 42% |
-
-
64598-64-7
N-D-ribityl-4,5-dimethyl-1,2-phenylenediamine

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| With oxygen In water at 20℃; for 96h; | 8% |
| With potassium hexacyanoferrate(III) |

| Conditions | Yield |
|---|---|
| With 4-morpholineethanesulfonic acid In water at 37℃; pH=6; |

-
-
569343-11-9
N-(ribityl),N'-(Boc)-diamino-4,5-dimethylbenzene

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| Stage #1: N-(ribityl),N'-(Boc)-diamino-4,5-dimethylbenzene With hydrogenchloride In water Stage #2: With sodium hydroxide In water at 20℃; for 5h; pH=7 - 8; | |
| Multi-step reaction with 2 steps 1: hydrogenchloride / water / 3 h / 20 °C 2: oxygen / water / 96 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 82 percent / NaHCO3 / H2O; tetrahydrofuran / 12 h 2.1: 85 mg / H2 / Pd(OH)2 / methanol / 2 h 3.1: HCl / H2O 3.2: NaOH / H2O / 5 h / 20 °C / pH 7 - 8 View Scheme | |
| Multi-step reaction with 4 steps 1: sodium hydrogencarbonate / 1,4-dioxane; water / 3 h / 20 °C 2: sodium cyanoborohydride / methanol / 48 h / Inert atmosphere; Reflux 3: hydrogenchloride / water / 3 h / 20 °C 4: oxygen / water / 96 h / 20 °C View Scheme |
-
-
371158-46-2
(2-amino-4,5-dimethyl-phenyl)-carbamic acid tert-butyl ester

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 85 mg / H2 / Pd(OH)2 / methanol / 2 h 2.1: HCl / H2O 2.2: NaOH / H2O / 5 h / 20 °C / pH 7 - 8 View Scheme | |
| Multi-step reaction with 3 steps 1: sodium cyanoborohydride / methanol / 48 h / Inert atmosphere; Reflux 2: hydrogenchloride / water / 3 h / 20 °C 3: oxygen / water / 96 h / 20 °C View Scheme |

-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| In methanol at 20℃; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 0.17 h / 55 °C 2: nitric acid / dichloromethane / 2 h / 15 - 25 °C 3: hydrogen; palladium 10% on activated carbon / methanol / 10 h / 20 °C 4: 6 h / 110 °C View Scheme |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
127167-47-9
(E)-11-(2-chloroethylidene)-6,11-dihydrodibenzoxepin-2-carboxylic acid methyl ester

-
-
127165-95-1
(E)-11-<2-(5,6-dimethyl-1-benzimidazoyl)ethylidene>-6,11-dihydrodibenzoxepin-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide; toluene for 3h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With trimethylamine Ambient temperature; | 100% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dimethyl-1H-benzo[d]imida-zole With potassium hydroxide In ethanol at 20℃; for 0.25h; Schlenk technique; Inert atmosphere; Stage #2: 1-(halomethyl)-3,5-dimethylbenzene In ethanol for 25h; Reflux; Schlenk technique; Inert atmosphere; | 100% |
| Stage #1: 5,6-dimethyl-1H-benzo[d]imida-zole In ethanol at 20℃; for 1h; Inert atmosphere; Schlenk technique; Alkaline conditions; Stage #2: 1-(halomethyl)-3,5-dimethylbenzene In ethanol at 80℃; Schlenk technique; Inert atmosphere; | 93% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
77487-30-0
4'-(5,6-Dimethyl-benzoimidazol-1-yl)-2,6,3'-trimethyl-4'H-[1,1']bipyridinyl-4-one

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol; acetonitrile at 20℃; for 0.5h; | 99% |

| Conditions | Yield |
|---|---|
| In toluene at 60℃; for 24h; Inert atmosphere; Schlenk technique; Glovebox; | 99% |

| Conditions | Yield |
|---|---|
| With potassium phosphate In N,N-dimethyl-formamide at 150℃; | 99% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
118-00-3
G

-
-
13082-84-3
(β-D-ribofuranosyl)-5,6-dimethylbenzimidazole

| Conditions | Yield |
|---|---|
| for 1.5h; purine nucleoside phosphorylase of whole cells of E. coli BMT 1D/1A; | 98% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
961-07-9
2'-Deoxyguanosine

-
-
4600-72-0
1-(5,6-dimethyl-benzoimidazol-1-yl)-β-D-erythro-1,2-dideoxy-pentofuranose

| Conditions | Yield |
|---|---|
| for 1.5h; purine nucleoside phosphorylase of whole cells of E. coli BMT 1D/1A; | 98% |

| Conditions | Yield |
|---|---|
| With iodine; potassium carbonate In N,N-dimethyl-formamide at 80℃; regioselective reaction; | 98% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
21472-08-2
5,6-Dimethyl-n-nitrobenzimidazol

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dimethyl-1H-benzo[d]imida-zole With tert-butyl methyl ether; nitric acid In tetrahydrofuran at 0℃; Stage #2: With sulfuric acid In dichloromethane at 0 - 5℃; for 1h; Further stages.; | 97% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
75-26-3
isopropyl bromide

-
-
1435268-00-0
1,3-diisopropyl-(5,6-dimethyl)benzimidazolium bromide

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dimethyl-1H-benzo[d]imida-zole With potassium carbonate In acetonitrile at 20℃; for 1h; Stage #2: isopropyl bromide In acetonitrile for 96h; Reflux; | 97% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
286-20-4
cyclohexane-1,2-epoxide

-
-
1184249-74-8
1-(2-hydroxycyclohexyl)-5,6-dimethylbenzimidazole

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 70℃; for 12h; | 97% |
| In neat (no solvent) at 70℃; for 12h; | 78% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
1178125-92-2
2-bromo-N-(4-methoxyphenyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| With copper(l) iodide; oxygen; sodium methylate; L-proline In N,N-dimethyl-formamide at 130℃; for 24h; Schlenk technique; Sealed tube; | 97% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dimethyl-1H-benzo[d]imida-zole With potassium hydroxide In ethanol at 20℃; for 1h; Inert atmosphere; Schlenk technique; Stage #2: (2,3,4,5,6-pentamethylbenzyl) halide In ethanol at 80℃; Inert atmosphere; Schlenk technique; | 97% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dimethyl-1H-benzo[d]imida-zole In ethanol at 20℃; for 1h; Inert atmosphere; Schlenk technique; Alkaline conditions; Stage #2: 1-(halomethyl)-2,3,4,5,6-pentamethylbenzene In ethanol at 80℃; Schlenk technique; Inert atmosphere; | 97% |
| Stage #1: 5,6-dimethyl-1H-benzo[d]imida-zole With potassium hydroxide In ethanol at 20℃; for 1h; Stage #2: 1-(halomethyl)-2,3,4,5,6-pentamethylbenzene In ethanol at 80℃; for 24h; |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; copper(l) chloride In chlorobenzene at 110℃; | 97% |
| With di-tert-butyl peroxide; copper(l) chloride In chlorobenzene at 110℃; for 2h; | 77% |

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dimethyl-1H-benzo[d]imida-zole With potassium hydroxide In ethanol at 20℃; for 0.25h; Stage #2: 2,3,4,5,6-pentamethylbenzyl chloride In ethanol at 20℃; for 17h; Reflux; | 97% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
50893-53-3
carbonochloridic acid 1-chloro-ethyl ester

-
-
24850-33-7
allyltributylstanane

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane 0 deg C to room temp., 3 h; | 96% |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; oxygen; sodium methylate; L-proline In N,N-dimethyl-formamide at 130℃; for 24h; Schlenk technique; Sealed tube; | 96% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
1183123-34-3
2-bromo-N-p-tolylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With copper(l) iodide; oxygen; sodium methylate; L-proline In N,N-dimethyl-formamide at 130℃; for 24h; Schlenk technique; Sealed tube; | 96% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
62802-42-0
2-chloro-5-fluoropyrimidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 70℃; for 24h; Inert atmosphere; | 96% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dimethyl-1H-benzo[d]imida-zole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.0833333h; Inert atmosphere; Stage #2: 6-(chloromethyl)-2-(methylthio)benzo[d]thiazole In N,N-dimethyl-formamide; mineral oil at 20℃; for 1h; | 96% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
17573-84-1
2,5-difluoropyrimidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 70℃; for 24h; Schlenk technique; | 96% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene In 1,2-dichloro-ethane at 20 - 80℃; for 6h; Schlenk technique; | 96% |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
74-88-4
methyl iodide

-
-
1128-27-4
1,5,6-trimethylbenzimidazole

| Conditions | Yield |
|---|---|
| With potassium hydroxide; 18-crown-6 ether In tetrachloromethane for 30h; Ambient temperature; | 95% |
| With sodium hydroxide In water; acetonitrile at 0 - 20℃; for 72h; Inert atmosphere; | 65% |
| Stage #1: 5,6-dimethyl-1H-benzo[d]imida-zole With sodium hydroxide In tetrahydrofuran for 0.166667h; Stage #2: methyl iodide In tetrahydrofuran at 20℃; | |
| Stage #1: 5,6-dimethyl-1H-benzo[d]imida-zole With sodium hydride In mineral oil at 0 - 20℃; for 0.5h; Schlenk technique; Stage #2: methyl iodide In mineral oil at 20℃; Schlenk technique; |
-
-
582-60-5
5,6-dimethyl-1H-benzo[d]imida-zole

-
-
10351-75-4
1H-benzimidazole-5,6-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate In water; tert-butyl alcohol at 70℃; for 0.5h; | 95% |
| With potassium permanganate In tert-butyl alcohol at 75℃; for 1.5h; | 60% |
| With potassium permanganate at 75℃; | 55% |
| With potassium permanganate In water; tert-butyl alcohol at 70℃; | 48% |
5,6-Dimethylbenzimidazole Consensus Reports
5,6-Dimethylbenzimidazole Specification
The 5,6-Dimethylbenzimidazole, with the CAS registry number 582-60-5, is also known as 1H-Benzimidazole, 5,6-dimethyl-. It belongs to the product categories of Benzimidazole; Imidazol & Benzimidazole; Benzimidazoles; Building Blocks; Heterocyclic Building Blocks. Its EINECS registry number is 209-488-1. This chemical's molecular formula is C9H10N2 and molecular weight is 146.19. Its IUPAC name is called 5,6-dimethyl-1H-benzimidazole. What's more, this chemical is mainly used for synthesis of vitamin B12. When you are using this chemical, please be cautious about it. You should avoid contact with skin and eyes. The product should be sealed and stored in cool and dry place.
Physical properties of 5,6-Dimethylbenzimidazole: (1)ACD/LogP: 2.31; (2)ACD/LogD (pH 5.5): 1.37; (3)ACD/LogD (pH 7.4): 2.26; (4)ACD/BCF (pH 5.5): 3.85; (5)ACD/BCF (pH 7.4): 30.27; (6)ACD/KOC (pH 5.5): 49.45; (7)ACD/KOC (pH 7.4): 389.15; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)Index of Refraction: 1.644; (11)Molar Refractivity: 46.26 cm3; (12)Molar Volume: 127.6 cm3; (13)Surface Tension: 50.4 dyne/cm; (14)Density: 1.145 g/cm3; (15)Flash Point: 208.6 °C; (16)Enthalpy of Vaporization: 60 kJ/mol; (17)Boiling Point: 376.8 °C at 760 mmHg; (18)Vapour Pressure: 1.53E-05 mmHg at 25°C.
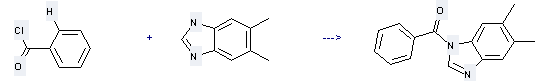
Uses of 5,6-Dimethylbenzimidazole: it can be used to produce 1-benzoyl-5,6-dimethyl-1H-benzoimidazole with benzoyl chloride at ambient temperature. This reaction will need reagent triethylamine and solvent CHCl3 with reaction time of 1 hour. The yield is about 82%.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC1=CC2=C(C=C1C)N=CN2
(2)InChI: InChI=1S/C9H10N2/c1-6-3-8-9(4-7(6)2)11-5-10-8/h3-5H,1-2H3,(H,10,11)
(3)InChIKey: LJUQGASMPRMWIW-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 400mg/kg (400mg/kg) | Russian Pharmacology and Toxicology Vol. 41, Pg. 249, 1978. |
Related Products
- 5-0185 (Beilstein Handbook Reference) 6850-22-2
- 5,10,15,20-Tetra(4-methylphenyl)-21H,23H-porphine
- 5,10,15,20-Tetra(4-pyridyl)porphyrin
- 5,10,15,20-Tetrakis(2,4,6-trimethylphenyl)-21H,23H-porphine
- 5,10,15,20-Tetrakis(4-hydroxyphenyl)porphyrin
- 5,10,15,20-Tetrakis(4-methoxyphenyl)-21H,23H-porphine
- 5,10,15,20-Tetrakis(N-methyl-4-pyridyl)porphine tetratosylate
- 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin
- 5,10,15,20-Tetraphenyl-21H,23H-porphine cobalt(II)
- 5,10,15,20-Tetraphenyl-21H,23H-porphine iron(III) chloride
- 58260-69-8
- 58260-71-2
- 58261-88-4
- 58261-91-9
- 582-62-7
- 58263-53-9
- 5826-73-3
- 5826-75-5
- 58267-85-9
- 582-69-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View