-
Name
5-Aminoindole
- EINECS 225-977-2
- CAS No. 5192-03-0
- Article Data109
- CAS DataBase
- Density 1.268 g/cm3
- Solubility insoluble in water
- Melting Point 131-133 °C (dec.)(lit.)
- Formula C8H8N2
- Boiling Point 354 °C at 760 mmHg
- Molecular Weight 132.165
- Flash Point 195 °C
- Transport Information
- Appearance Grey Crystals
- Safety 26-45-36/37
- Risk Codes 22-36/37/38-68
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms Indol-5-ylamine;Indole, 5-amino-;1H-Indol-5-amine;5-Amino indole;5-Amino-1H-indole;
- PSA 41.81000
- LogP 2.33130
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrazine hydrate at 90℃; for 1.5h; chemoselective reaction; | 99% |
| With C20H32Cl4Cu2N4O4 In water at 60℃; for 2h; Inert atmosphere; Schlenk technique; | 99% |
| With ammonia borane; cetyltrimethylammonim bromide; [Ru(p-cymene)(2,6-bis(1-methylimidazole-2-thione)pyridine)](PF6)2 In water; acetonitrile at 79.84℃; for 24h; Inert atmosphere; Schlenk technique; | 99% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; 5%-palladium/activated carbon; hydrazine hydrate; lithium hydroxide In 1,4-dioxane at 170℃; for 16h; Molecular sieve; Inert atmosphere; | 90% |
-
-
166104-20-7
5-aminoindole-1-carboxylic acid tert-butyl ester

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol; water for 4h; Heating; | 85% |
| In 2,2,2-trifluoroethanol at 150℃; for 0.25h; microwave irradiation; | 81% |
| With 2,2,2-trifluoroethanol at 150℃; for 0.25h; Product distribution / selectivity; Microwave irradiation; | 81% |
| With 2,2,2-trifluoroethanol at 150℃; for 0.25h; Product distribution / selectivity; Microwave irradiation; | 81% |
| With 1,1,1,3',3',3'-hexafluoro-propanol at 150℃; for 0.25h; Product distribution / selectivity; Microwave irradiation; | 70% |
-
-
32692-19-6
5-nitro-2,3-dihydro-1H-indole

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium tert-butylate; oxygen; nickel dibromide In tert-Amyl alcohol at 95℃; for 48h; | 85% |
| Multi-step reaction with 3 steps 1: 91 percent / Br2 / acetic acid 2: 91 percent / 2,3-dichloro-5,6-dicyanobenzoquinone / benzene / 6 h / Heating 3: 54 percent / hydrogen, NaOAc*3H2O / Raney nickel / ethanol / 1 h / 2327.2 Torr / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: tetrachloro-<1,4>benzoquinone; xylene 2: N2H4+H2O; Raney nickel; ethanol View Scheme |
-
-
10075-50-0
5-bromo-1H-indole

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper(I) iodide; 1-ethylacetoacetate-3-methyl imidazolium hydroxide In acetonitrile at 80℃; for 12h; Inert atmosphere; | 82% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 25℃; Substitution; Electrolysis; | A 15% B 70% |

-
-
33632-27-8
1-(5-nitro-2,3-dihydroindol-1-yl)ethanone

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| With sodium hydroxide; aluminium-nickel alloy In water for 1.5h; | 56% |
| Multi-step reaction with 3 steps 1: aq.-ethanolic hydrochloric acid 2: tetrachloro-<1,4>benzoquinone; xylene 3: N2H4+H2O; Raney nickel; ethanol View Scheme |
-
-
87240-07-1
7-bromo-5-nitro-1H-indole

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| With hydrogen; sodium acetate; nickel In ethanol under 2327.2 Torr; for 1h; Ambient temperature; | 54% |
-
-
37877-90-0
5-phenylazoindole

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| With tin(ll) chloride |

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 25℃; Product distribution; Further Variations:; Reagents; Reduction; substitution; Electrolysis; | A 12 % Chromat. B 14 % Chromat. |

-
-
166104-19-4
5-nitroindole-1-carboxylic acid tert-butyl ester

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NH2NH2*H2O / Pd-C / methanol / Heating 2: 85 percent / K2CO3 / methanol; H2O / 4 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: NaOH, Na2CO3 / H2O / 1 h / 0 - 5 °C 2: chloranil / xylene / 4 h / 110 - 150 °C 3: stannous chloride View Scheme | |
| Multi-step reaction with 3 steps 1: acetic acid anhydride; nitric acid / 10 °C / Erhitzen des Reaktionsprodukts mit konz. wss. Salzsaeure 2: tetrachloro-<1,4>benzoquinone; xylene 3: N2H4+H2O; Raney nickel; ethanol View Scheme |
-
-
37867-81-5
3-(N-indolino)-1-phenyltriazene

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: chloranil / xylene / 4 h / 110 - 150 °C 2: stannous chloride View Scheme |
-
-
87240-06-0
7-bromo-5-nitroindoline

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / 2,3-dichloro-5,6-dicyanobenzoquinone / benzene / 6 h / Heating 2: 54 percent / hydrogen, NaOAc*3H2O / Raney nickel / ethanol / 1 h / 2327.2 Torr / Ambient temperature View Scheme |
-
-
16078-30-1
1-Acetyl-2,3-dihydro-1H-indole

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 77 percent / nitric acid (fuming) / acetic acid / 1.) 9-12 deg C, 2.) room temp., 1.5 h 2: 56 percent / aluminium-nickel alloy, NaOH / H2O / 1.5 h View Scheme |
-
-
16730-20-4
5-nitro-1H-indole-2-carboxylic acid

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: copper (II)-oxide; quinoline 2: platinum; ethanol / Hydrogenation View Scheme |
-
-
16732-57-3
5-nitro-indole-2-carboxylic acid ethyl ester

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aq.-ethanolic KOH 2: copper (II)-oxide; quinoline 3: platinum; ethanol / Hydrogenation View Scheme |
-
-
73647-04-8
ethyl 2-[2-(4-nitrophenyl)hydrazinylidene]propanoate

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: polyphosphoric acid 2: aq.-ethanolic KOH 3: copper (II)-oxide; quinoline 4: platinum; ethanol / Hydrogenation View Scheme |
-
-
1445-73-4
1-Methyl-4-piperidone

-
-
116480-62-7
3-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-1H-indol-5-amine

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol | 1.11 gm (48.9%) |


| Conditions | Yield |
|---|---|
| Stage #1: 5-nitroindole With hydrogen; palladium on activated carbon In ethyl acetate at 20℃; under 2280.15 Torr; for 1.5h; Stage #2: N-benzyliminodipropionic acid With 1,1'-carbonyldiimidazole In tetrahydrofuran for 3h; Heating / reflux; Stage #3: With lithium aluminium tetrahydride; sulfuric acid; ammonium formate; palladium on activated carbon more than 3 stages; |

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| With hydrogenchloride In chloroform; water for 1h; | 32.7 mg |
-
-
5192-03-0
5-amino-1H-indole

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
184031-16-1
(1H-indol-5-yl)-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20℃; for 24h; | 100% |
| In ethyl acetate at 20℃; for 12h; | 95% |
| With triethylamine In methanol at 20℃; for 6h; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-amino-1H-indole; Glycine ethyl ester isocyanate In ethanol for 2h; Stage #2: With hydrogenchloride In ethanol for 3h; Heating; | 99.3% |
-
-
5192-03-0
5-amino-1H-indole

-
-
594-44-5
Ethanesulfonyl chloride

-
-
141101-59-9
5-ethanesulfonylamino-1H-indole

| Conditions | Yield |
|---|---|
| With hydrogenchloride; triethylamine In dichloromethane; water; ethyl acetate | 99% |
| With hydrogenchloride; triethylamine In dichloromethane; water; ethyl acetate | 99% |
| With hydrogenchloride; triethylamine In dichloromethane; water; ethyl acetate | 99% |
-
-
5192-03-0
5-amino-1H-indole

-
-
141823-14-5
4-((6-bromohexyl)oxy)benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 5-amino-1H-indole; 4-((6-bromohexyl)oxy)benzaldehyde In 1,2-dichloro-ethane at 20℃; for 0.5h; Stage #2: With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; for 24h; | 99% |
| Stage #1: 5-amino-1H-indole; 4-((6-bromohexyl)oxy)benzaldehyde In 1,2-dichloro-ethane at 20℃; for 0.5h; Stage #2: With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 20℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 20h; | 99% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 20h; | 99% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 20h; | 99% |
-
-
5192-03-0
5-amino-1H-indole

-
-
33904-04-0
4-isothiocyanato-1,2-dimethoxybenzene

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 20h; | 99% |
-
-
5192-03-0
5-amino-1H-indole

-
-
152120-54-2, 862686-58-6, 1143572-00-2
N,N'-bis( tert-butoxycarbonyl)-1H-pyrazole-1-carboxamidine

| Conditions | Yield |
|---|---|
| With chloroform In neat (no solvent) for 4h; Milling; | 99% |
-
-
5192-03-0
5-amino-1H-indole

-
-
83135-00-6
(hex-1-en-3-yl)methyl carbonate

| Conditions | Yield |
|---|---|
| With cobalt(II) tetrafluoroborate; C36H29N2O2P; zinc In acetonitrile at 20℃; for 16h; Inert atmosphere; Sealed tube; enantioselective reaction; | 99% |
-
-
5192-03-0
5-amino-1H-indole

-
-
22445-41-6
3,5-dimethylphenyl iodide

-
-
360045-07-4
1-(3,5-dimethylphenyl)-5-aminoindole

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; (S,S)-1,2-diaminocyclohexane In 1,4-dioxane; dodecane at 110℃; for 24h; | 98% |
-
-
5192-03-0
5-amino-1H-indole

-
-
4489-34-3
4,6-dichloro-2-(methylsulfonyl)pyrimidine

-
-
1450821-85-8
N-[6-chloro-2-(methylsulfonyl)pyrimidin-4-yl]-1H-indol-5-amine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dimethyl sulfoxide at 20℃; for 1h; Inert atmosphere; chemoselective reaction; | 98% |
-
-
5192-03-0
5-amino-1H-indole

-
-
39263-34-8
N′-(2-cyano-4-nitrophenyl)-N,N-dimethylformimidamide

| Conditions | Yield |
|---|---|
| With acetic acid at 115℃; | 97.47% |
| With acetic acid for 3h; Reflux; | 80% |
-
-
1193-21-1
4,6-dichloropyrimidine

-
-
5192-03-0
5-amino-1H-indole

-
-
670252-90-1
N-(6-chloropyrimidin-4-yl)-1H-indol-5-amine

| Conditions | Yield |
|---|---|
| With triethylamine In isopropyl alcohol at 20℃; for 1h; | 97.4% |
| With 1-methyl-pyrrolidin-2-one; N-ethyl-N,N-diisopropylamine at 50℃; for 2.5h; | 38% |
| With triethylamine In isopropyl alcohol at 20℃; for 2h; | |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 20℃; for 12h; |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran | 97% |
| With benzene |
-
-
159768-48-6
4-chloro-7-fluoro-6-methoxyquinazoline

-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| In isopropyl alcohol | 97% |
-
-
5192-03-0
5-amino-1H-indole

-
-
7693-46-1
4-Nitrophenyl chloroformate

-
-
163487-27-2
(1H-indol-5-yl)-carbamic acid 4-nitro-phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 2h; Inert atmosphere; | 97% |
| With triethylamine In dichloromethane at 20℃; for 2h; Inert atmosphere; | |
| With triethylamine In dichloromethane at 20℃; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; diethyl ether; N,N-dimethyl-formamide at 40℃; for 4h; | 97% |
-
-
5192-03-0
5-amino-1H-indole

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 20h; | 97% |
-
-
5192-03-0
5-amino-1H-indole

-
-
898252-84-1
2-butyl-4-chloro-5-phenylisothiazol-3(2H)-one 1,1-dioxide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 140℃; for 0.416667h; Microwave irradiation; | 96.5% |
-
-
5192-03-0
5-amino-1H-indole

-
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-
-
856935-80-3
4-(1H-indol-5-ylamino)-piperidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 5-amino-1H-indole; N-tert-butyloxycarbonylpiperidin-4-one With sodium tris(acetoxy)borohydride; acetic acid In 1,2-dichloro-ethane at 20℃; for 4h; Stage #2: With sodium hydroxide In water; 1,2-dichloro-ethane | 96% |
| With sodium tris(acetoxy)borohydride; acetic acid In acetonitrile at 20℃; for 1h; | |
| With sodium tris(acetoxy)borohydride; acetic acid In 1,2-dichloro-ethane at 20℃; for 18h; |
-
-
5192-03-0
5-amino-1H-indole

-
-
606-23-5
1H-indene-1,3(2H)-dione

-
-
135-02-4
ortho-anisaldehyde

-
-
1449762-94-0
12-(2-methoxyphenyl)indeno[1,2-b]pyrrolo[3,2-f]quinolin-11(3H)-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 8h; Reflux; | 96% |

-
-
5192-03-0
5-amino-1H-indole

-
-
5973-71-7
3,4-dimethylbenzaldehyde

-
-
126-81-8
dimedone

-
-
1449762-71-3
8,9-dihydro-8,8-dimethyl-11-(3,4-dimethylphenyl)-3H-pyrrolo[3,2-a]acridin-10(6H,7H,11H)-one

| Conditions | Yield |
|---|---|
| In ethanol for 10h; Reflux; | 96% |

-
-
5192-03-0
5-amino-1H-indole

-
-
126-81-8
dimedone

-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
1449762-72-4
8,9-dihydro-11-(3,4-dimethoxyphenyl)-8,8-dimethyl-3H-pyrrolo[3,2-a]acridin-10(6H,7H,11H)-one

| Conditions | Yield |
|---|---|
| In ethanol for 10h; Reflux; | 96% |


| Conditions | Yield |
|---|---|
| scandium tris(trifluoromethanesulfonate) at 20℃; for 2h; modified Skraup reaction; | 95% |

| Conditions | Yield |
|---|---|
| scandium tris(trifluoromethanesulfonate) In acetonitrile at 20℃; for 6h; modified Skraup reaction; | A 2% B 95% |


| Conditions | Yield |
|---|---|
| In ethanol for 10h; Reflux; | 95% |

-
-
5192-03-0
5-amino-1H-indole

-
-
606-23-5
1H-indene-1,3(2H)-dione

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
1449762-90-6
12-(3-methoxyphenyl)indeno[1,2-b]pyrrolo[3,2-f]quinolin-11(3H)-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 10h; Reflux; | 95% |

5-Aminoindole Specification
The CAS register number of 5-Aminoindole is 5192-03-0. It also can be called as Indole, 5-amino- and the IUPAC name about this chemical is 1H-indol-5-amine. The molecular formula about this chemical is C8H8N2 and the molecular weight is 132.16. It belongs to the following product categories, such as Indole Derivative; Indoles and derivatives; IndoleDerivative; Indoles; Indole Derivatives; Simple Indoles and so on. This chemical is stable under normal temperature and pressure. If you want to store it, the storage temperature must be at 0-6 °C, and it need keep in a dark, cool and dry place.
Physical properties about 5-Aminoindole are: (1)ACD/LogP: 0.70; (2)ACD/LogD (pH 5.5): 0.58; (3)ACD/LogD (pH 7.4): 0.7; (4)ACD/BCF (pH 5.5): 1.53; (5)ACD/BCF (pH 7.4): 2; (6)ACD/KOC (pH 5.5): 43.67; (7)ACD/KOC (pH 7.4): 57.06; (8)#H bond acceptors: 2; (9)#H bond donors: 3; (10)#Freely Rotating Bonds: 1; (11)Polar Surface Area: 8.17Å2; (12)Index of Refraction: 1.757; (13)Molar Refractivity: 42.76 cm3; (14)Molar Volume: 104.1 cm3; (15)Polarizability: 16.95x10-24cm3; (16)Surface Tension: 65.7 dyne/cm; (17)Enthalpy of Vaporization: 59.89 kJ/mol; (18)Boiling Point: 354 °C at 760 mmHg; (19)Vapour Pressure: 3.46E-05 mmHg at 25°C.
Preparation: this chemical can be prepared by 5-nitro-indole. This reaction will need reagent N2H4+H2O, Raney nickel and ethanol.
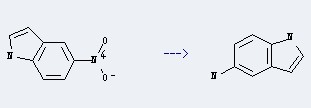
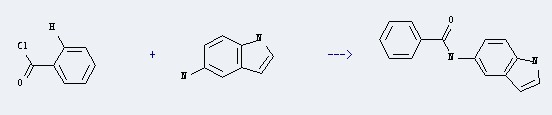
Uses of 5-Aminoindole: it can be used to produce N-indol-5-yl-benzamide with benzoyl chloride. This reaction will need reagent aqueous alkali and benzene.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful if swallowed and it is irritating to eyes, respiratory system and skin, it has possible risk of irreversible effects. When you are using it, wear suitable protective clothing and gloves. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice and in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc2c(cc[nH]2)cc1N
(2)InChI: InChI=1/C8H8N2/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H,9H2
(3)InChIKey: ZCBIFHNDZBSCEP-UHFFFAOYAC
(4)Std. InChI: InChI=1S/C8H8N2/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H,9H2
(5)Std. InChIKey: ZCBIFHNDZBSCEP-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View