-
Name
5-Aminoisoquinoline
- EINECS 214-408-3
- CAS No. 1125-60-6
- Article Data59
- CAS DataBase
- Density 1.21g/cm3
- Solubility
- Melting Point 127-130 °C
- Formula C9H8N2
- Boiling Point 335.7 °C at 760 mmHg
- Molecular Weight 144.176
- Flash Point 183.3 °C
- Transport Information
- Appearance yellow to brown crystalline powder
- Safety 36/37/39-26-37/39-36
- Risk Codes 38-20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms 5-Amino-isoquinoline;Isoquinoline, 5-amino- (8CI);5-Isoquinolinamine;isoquinolin-5-amine;Isoquinoline, 5-amino-;Isoquinol-5-ylamine;5-Amino Isoquinoline;
- PSA 38.91000
- LogP 2.39820
Synthetic route

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In ethanol for 1h; Heating; | 99% |
| With palladium 10% on activated carbon; hydrogen at 20℃; for 1h; | 99% |
| With indium; ammonium chloride In ethanol Heating; | 99% |
-
-
34784-04-8
5-bromoisoquinoline

-
-
1125-60-6
isoquinolin-5-ylamine

| Conditions | Yield |
|---|---|
| With 2-((dicyclohexylphosphino)methyl)-1,3-bis(2,6-diisopropylphenyl)-4,5-dimethyl-1H-imidazol-3-ium iodide; ammonia; palladium diacetate; sodium t-butanolate In 1,4-dioxane at 120℃; under 7500.75 Torr; for 24h; Autoclave; Inert atmosphere; | 99% |
| With lithium amide; (CyPF-t-Bu)PdCl2 In 1,2-dimethoxyethane at 90℃; for 24h; | 79% |
| With ammonia; palladium diacetate; sodium t-butanolate; 2-(di-tert-butyl-phosphino)-1-phenyl-1H-pyrrole In 1,4-dioxane at 120℃; under 7500.75 Torr; for 24h; Autoclave; Inert atmosphere; | 78 %Chromat. |
-
-
57554-78-6
5-Nitroisoquinoline N-Oxide

-
-
1125-60-6
isoquinolin-5-ylamine

| Conditions | Yield |
|---|---|
| With titanium(III) chloride In acetic acid at 18℃; for 0.116667h; | 97.8% |
| With hydrogenchloride; palladium on activated charcoal Hydrogenation; |
-
-
58142-72-6
5-nitroisoquinoline hydrobromide

-
-
1125-60-6
isoquinolin-5-ylamine

| Conditions | Yield |
|---|---|
| With titanium(III) chloride In acetic acid at 20℃; for 0.116667h; | 86.8% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromoisoquinoline With lithium amide; [(CyPF-tBu)PdCl2] In 1,2-dimethoxyethane at 90℃; for 24h; Sealed vial; Stage #2: With hydrogenchloride In 1,2-dimethoxyethane; water at 20℃; for 0.0833333h; Stage #3: With sodium hydrogencarbonate In 1,2-dimethoxyethane; water Product distribution / selectivity; | A 79% B n/a |
| Stage #1: 5-bromoisoquinoline With ammonia; sodium t-butanolate; [(CyPF-tBu)PdCl2] In 1,2-dimethoxyethane at 90℃; under 4137.29 - 10343.2 Torr; for 20h; Stage #2: With hydrogenchloride In 1,2-dimethoxyethane; water at 20℃; for 0.0833333h; Stage #3: With sodium hydrogencarbonate In 1,2-dimethoxyethane; water Product distribution / selectivity; | A 70% B n/a |

| Conditions | Yield |
|---|---|
| With ammonium sulfate; C39H45FeNNiP2; sodium t-butanolate In 2-methyltetrahydrofuran at 100℃; for 7h; Reagent/catalyst; Solvent; Inert atmosphere; Glovebox; Autoclave; | 75% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; 5%-palladium/activated carbon; hydrazine hydrate; lithium hydroxide In 1,4-dioxane at 170℃; for 16h; Molecular sieve; Inert atmosphere; | 30% |
| With ammonium hydroxide; sulfur dioxide at 150 - 160℃; |
-
-
374554-54-8
5-amino-1-chloroisoquinoline

-
-
1125-60-6
isoquinolin-5-ylamine

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate; sodium hydroxide; nickel Hydrogenation; |
-
-
57554-78-6
5-Nitroisoquinoline N-Oxide

-
A
-
1125-60-6
isoquinolin-5-ylamine

-
B
-
115955-90-3
1,2,3,4-tetrahydroisoquinolin-5-amine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; palladium on activated charcoal Hydrogenation; |
-
-
57554-78-6
5-Nitroisoquinoline N-Oxide

-
A
-
1125-60-6
isoquinolin-5-ylamine

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; ethanol Hydrogenation; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: KNO3, H2SO4, aq. Na2CO3 / 25 °C 2: H2 / Pd-C / methanol View Scheme | |
| Multi-step reaction with 2 steps 1.1: potassium nitrate; sulfuric acid / -15 - 20 °C 1.2: 0 °C / pH 8 2.1: ammonium chloride; iron / ethanol; water / 10 h / 80 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid; potassium nitrate / 2 h / -15 - 20 °C 2: iron; ammonium chloride / ethanol; water / 10 h / 20 - 80 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid; potassium nitrate / 2 h / -15 - 20 °C 2: ammonium chloride; iron / ethanol; water / 10 h / 20 - 80 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium nitrate; sulfuric acid / 2 h / 0 °C 2: tin(ll) chloride; hydrogenchloride / water / 1 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 86 percent / conc. H2SO4, KNO3 / 6 h / 50 °C 2: 79 percent / activated carbon, FeCl3*6H2O, hydrazine / methanol / 16 h / Heating View Scheme |

-
-
58142-97-5
1-chloro-5-nitroisoquinoline

-
-
1125-60-6
isoquinolin-5-ylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Raney nickel / Hydrogenation 2: Raney nickel; ethanolic NaOH; H2ptcl6 / Hydrogenation View Scheme |
-
-
19493-44-8
1-chloroisoquinoline

-
-
1125-60-6
isoquinolin-5-ylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: concentrated sulfuric acid; potassium nitrate 2: Raney nickel / Hydrogenation 3: Raney nickel; ethanolic NaOH; H2ptcl6 / Hydrogenation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: concentrated sulfuric acid; potassium nitrate 2: palladium/charcoal; aq.-ethanolic HCl / Hydrogenation View Scheme | |
| Multi-step reaction with 2 steps 1: concentrated sulfuric acid; potassium nitrate 2: palladium/charcoal; aq.-ethanolic HCl / Hydrogenation View Scheme | |
| Multi-step reaction with 2 steps 1: concentrated sulfuric acid; potassium nitrate 2: palladium/charcoal; ethanol / Hydrogenation View Scheme |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
77958-57-7
hexakis(4-formylphenoxy)cyclotriphosphazene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 99% |
| In pyridine at 20℃; | 94% |
| for 20h; Ambient temperature; | 80% |
-
-
17347-61-4
2,2-dimethylsuccinic anhydride

-
-
1125-60-6
isoquinolin-5-ylamine

-
-
1360590-33-5
1-(isoquinolin-5-yl)-3,3-dimethylpyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| at 150℃; neat (no solvent); | 99% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
532-55-8
Benzoyl isothiocyanate

-
-
72677-81-7
1-benzoyl-3-isoquinolin-5-yl-thiourea

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 1h; | 98% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
1885-14-9
phenyl chloroformate

-
-
628721-45-9
(isoquinolin-5-yl)carbamic acid phenyl ester

| Conditions | Yield |
|---|---|
| Stage #1: isoquinolin-5-ylamine With pyridine In acetonitrile at 20℃; Stage #2: phenyl chloroformate In acetonitrile at 20℃; for 1h; | 97% |
| With sodium hydrogencarbonate In dichloromethane | 92% |
| at 0 - 20℃; | 72% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
43101-10-6
5-azidoisoquinoline

| Conditions | Yield |
|---|---|
| Stage #1: isoquinolin-5-ylamine With sulfuric acid In water at 0℃; for 0.0833333h; Stage #2: With sodium azide In water at 20℃; for 0.166667h; | 96.9% |
| Stage #1: isoquinolin-5-ylamine With hydrogenchloride; sodium nitrite In water at 0℃; for 1h; Stage #2: With sodium azide; sodium acetate In water at 20℃; for 15h; | 95% |
| With hydrogenchloride; sodium azide; sodium acetate; sodium nitrite 1.) water, 5 deg C; Yield given. Multistep reaction; | |
| Stage #1: isoquinolin-5-ylamine With hydrogenchloride; sodium nitrite In water at 0 - 5℃; for 0.5h; Stage #2: With sodium azide In water at 0 - 20℃; |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
1840-19-3
1-isothiocyanato-3-trifluoromethyl-benzene

-
-
1356927-94-0
1-(isoquinolin-5-yl)-3-(3-(trifluoromethyl)phenyl)thiourea

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; toluene at 60℃; | 96% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
6590-93-8
3,5-dichlorophenylisothiocyanate

-
-
1356927-95-1
1-(3,5-dichlorophenyl)-3-(isoquinolin-5-yl)thiourea

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; toluene at 60℃; | 96% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
50-00-0
formaldehyd

-
-
542-92-7
cyclopenta-1,3-diene

-
-
138495-23-5
(6aS,9aR)-6,6a,7,9a-Tetrahydro-5H-cyclopenta[c][1,8]phenanthroline

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In water; acetonitrile for 1h; Ambient temperature; | 95% |
| With trifluoroacetic acid In acetonitrile for 0.166667h; Ambient temperature; | 95% |

-
-
688-61-9, 53588-88-8, 90721-85-0, 151452-33-4, 151452-35-6
Triallylborane

-
-
1125-60-6
isoquinolin-5-ylamine

| Conditions | Yield |
|---|---|
| Stage #1: Triallylborane; isoquinolin-5-ylamine In benzene for 0.5h; Heating; Stage #2: With isopropyl alcohol for 2h; Heating; Stage #3: With sodium hydroxide | 95% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
15952-61-1
6-chloropiperonal

| Conditions | Yield |
|---|---|
| In various solvent(s) for 24h; Heating; | 95% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
123-62-6
propionic acid anhydride

-
-
1190561-61-5
N-(5-isoquinolinyl)propionamide

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 95% |
| With pyridine at 20℃; for 24h; Inert atmosphere; | 82% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
622-58-2
p-Tolylisocyanate

-
-
1356927-90-6
1-(isoquinolin-5-yl)-3-p-tolylurea

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; toluene at 60℃; | 95% |

| Conditions | Yield |
|---|---|
| With pyridine at 0℃; for 2h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium carbonate; sulfur In water at 80℃; for 5h; | 95% |
| Stage #1: carbon disulfide; isoquinolin-5-ylamine With triethylamine In tetrahydrofuran at 20℃; Stage #2: With dmap; di-tert-butyl dicarbonate In tetrahydrofuran at 0 - 20℃; | 100 %Chromat. |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
30806-83-8
p-ethoxycarbonylphenyl isocyanate

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane at 90℃; | 95% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 70 - 75℃; for 11h; Inert atmosphere; | 95% |
| In acetonitrile at 70 - 75℃; for 11h; Temperature; chemoselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| In methanol for 5h; Reflux; | 94% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 120℃; under 1500.15 Torr; for 0.166667h; Microwave irradiation; | 94% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 93% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
542-85-8
Ethyl isothiocyanate

-
-
140192-80-9
N-ethyl-N1-(5-isoquinolinyl)thiourea

| Conditions | Yield |
|---|---|
| In methanol for 10h; Reflux; | 92% |
| In tetrahydrofuran | 34% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
38985-64-7
o-fluorophenyl isothiocyanate

-
-
454194-73-1
1-(2-fluorophenyl)-3-(isoquinolin-5-yl)thiourea

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; toluene at 60℃; | 92% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
2131-61-5
p-nitrophenyl isothiocyanate

-
-
1095927-44-8
1-(isoquinolin-5-yl)-3-(4-nitrophenyl)thiourea

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; toluene at 60℃; | 92% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
101385-93-7
3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
1035096-80-0
N-(pyrrolidin-3-yl)isoquinolin-5-amine

| Conditions | Yield |
|---|---|
| Stage #1: isoquinolin-5-ylamine; 3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester With sodium tris(acetoxy)borohydride In acetic acid at 0 - 20℃; Stage #2: With hydrogenchloride In diethyl ether for 6h; | 92% |
-
-
1125-60-6
isoquinolin-5-ylamine

-
-
1376771-60-6
1-oxa-3,6-dithiacycloheptane

| Conditions | Yield |
|---|---|
| Stage #1: 1-oxa-3,6-dithiacycloheptane With samarium(III) nitrate hexahydrate In chloroform at 20℃; for 0.5h; Inert atmosphere; Stage #2: isoquinolin-5-ylamine In ethanol; chloroform at 20℃; for 3h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol at 25℃; for 2h; regioselective reaction; | 92% |
5-Aminoisoquinoline Chemical Properties
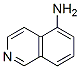
IUPAC Name: isoquinolin-5-amine
Molecular Formula:C9H8N2
Molecular Weight:144.173220 g/mol
Appearance:Light Yellow Solid
Density:1.21g/cm3
Melting Point:125-128 °C
Flash Point:183.3 °C
Sensitive:Light
EINECS:214-408-3
BRN:114465
Synonyms of 5-AMINO ISOQUINOLINE(1125-60-6):
5-Aminoisoquinoline;TIMTEC-BB SBB004127;ISOQUINOLIN-5-YLAMINE;5-AMINOISOQUINOLINE;Isoquinoline, 5-amino-;isoquinol-5-ylamine;isoquinolin-5-amine;5-Aminoisoquinoline ,99%;5-Isoquinolinamine
Categories of 5-AMINO ISOQUINOLINE(1125-60-6):
Amines;Quinolines, Isoquinolines & Quinoxalines;Quinoline&Isoquinoline;Aromatics Compounds;Aromatics;Quinolines, Isoquinolines & Quinoxalines;Building Blocks;Heterocyclic Building Blocks;Isoquinolines
5-Aminoisoquinoline Uses
5-Aminoisoquinoline Safety Profile
Hazard Codes:Xn,Xi

Risk Statements:38-20/21/22-36/37/38
38:Irritating to the skin
20/21/22:Harmful by inhalation, in contact with skin and if swallowed
36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements:36/37/39-26-37/39-36
36/37/39:Wear suitable protective clothing, gloves and eye/face protection
26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
37/39:Wear suitable gloves and eye/face protection
36:Wear suitable protective clothing
Hazard Note:Irritant
PackingGroup:III
5-Aminoisoquinoline Specification
First Aid Measures of 5-AMINO ISOQUINOLINE(1125-60-6):
Ingestion:Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Skin:Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Eyes:Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Storage of 5-AMINO ISOQUINOLINE(1125-60-6):
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Related Products
- 5-Aminoisoquinoline
- 112565-42-1
- 112566-17-3
- 1125-66-2
- 112568-12-4
- 112571-69-4
- 112-57-2
- 1125746-49-7
- 1125-78-6
- 1125-80-0
- 112581-74-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View