-
Name
5-Bromoisatin
- EINECS 201-747-7
- CAS No. 87-48-9
- Article Data106
- CAS DataBase
- Density 1.826 g/cm3
- Solubility
- Melting Point 251-253 °C
- Formula C8H4BrNO2
- Boiling Point 136ºC
- Molecular Weight 226.029
- Flash Point
- Transport Information UN 2811
- Appearance Orange crystalline powder
- Safety 26-36/39-37/39-36
- Risk Codes 37/38-41
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Indole-2,3-dione,5-bromo- (7CI,8CI);Isatin, 5-bromo- (6CI);5-Bromo-1H-indole-2,3-dione;5-Bromo-2,3-indolinedione;5-Bromoindole-2,3-dione;NSC 4980;
- PSA 46.17000
- LogP 1.72190
Synthetic route
-
-
66475-83-0
N-(4-bromophenyl)-2-(N-hydroximino)acetamide

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid at 60 - 80℃; for 0.166667h; | 96% |
| With sulfuric acid at 60 - 80℃; | 89% |
| With sulfuric acid at 70℃; for 0.00277778h; Microwave irradiation; | 88% |
-
-
20870-78-4
5-bromo-2-indolin-2-one

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) acetate monohydrate; potassium carbonate In N,N-dimethyl-formamide at 50℃; under 760.051 Torr; for 0.5h; | 96% |
| With tert.-butylnitrite; oxygen In tetrahydrofuran at 50℃; under 760.051 Torr; for 24h; Schlenk technique; Inert atmosphere; | 85% |
| With oxygen; sodium iodide In tetrahydrofuran at 60℃; for 12h; Schlenk technique; chemoselective reaction; | 83% |

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In dimethyl sulfoxide at 25 - 35℃; for 7h; | 94% |

| Conditions | Yield |
|---|---|
| With bromine; acetic acid at 0℃; | 89.43% |
| With tribromo-isocyanuric acid In trifluoroacetic acid at 20℃; for 0.5h; | 85% |
| With N-Bromosuccinimide In acetonitrile at 20℃; for 96h; | 77% |

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With copper(I) bromide In water; benzonitrile at 140℃; for 4h; Inert atmosphere; | 88% |
-
-
10075-50-0
5-bromo-1H-indole

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With pyridinium chlorochromate In 1,2-dichloro-ethane at 80℃; for 2.5h; | 87% |
| With indium(III) chloride; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In water; acetonitrile at 80℃; for 2h; | 86% |
| With N-iodo-succinimide; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In dimethyl sulfoxide at 15 - 25℃; for 3h; | 86% |
-
-
22190-33-6
5-bromoindoline

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With iodine pentoxide In dimethyl sulfoxide at 80℃; | 86% |
| With o-iodosobenzoic acid; oxygen In dimethyl sulfoxide at 80℃; for 12h; Green chemistry; | 78% |

| Conditions | Yield |
|---|---|
| With sodium chloride In water; acetonitrile at 50℃; for 2h; Green chemistry; | 85% |
-
-
182344-70-3
5-bromo-indole-1-carboxylic acid tert-butyl ester

-
B
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In dimethyl sulfoxide at 15 - 25℃; for 3h; | A 85% B 5% |

| Conditions | Yield |
|---|---|
| Stage #1: chloral hydrate; 4-bromo-aniline With sulfuric acid; hydroxylamine hydrochloride; sodium sulfate In water at 130℃; for 1h; Stage #2: With sulfuric acid at 50 - 70℃; for 1.5h; | 78% |
| With hydrogenchloride; hydroxylamine hydrochloride; sodium sulfate In water at 90℃; for 0.0833333h; | 67.2% |
| Stage #1: chloral hydrate; 4-bromo-aniline With hydrogenchloride; hydroxylamine hydrochloride; sodium sulfate In water Stage #2: With sulfuric acid at 60 - 80℃; | 67% |
-
-
17630-75-0
5-chloro-indolin-2-one

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite In tetrahydrofuran at 25℃; under 1520.1 Torr; | 74% |

| Conditions | Yield |
|---|---|
| Stage #1: chloral; 4-bromo-aniline With hydroxylamine hydrochloride; sodium sulfate In water at 80 - 90℃; for 2h; Stage #2: With sulfuric acid In water at 50 - 80℃; for 0.75h; | 70% |
-
-
1021235-39-1
N-ethyl-2-(p-tolylamino)acetamide

-
-
106-40-1
4-bromo-aniline

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With oxygen; (R,S)-2-chloropropionic acid In benzonitrile at 70℃; under 760.051 Torr; for 9h; | 54.5% |

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With Oxone In tetrahydrofuran; water at 20℃; for 0.166667h; Inert atmosphere; | 54% |
-
-
85976-21-2
N-(4-bromo-2-(2,2-dibromoacetyl)phenyl)acetamide

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With potassium permanganate | |
| Multi-step reaction with 2 steps 1: alcohol; water; hydrobromic acid / man faellt mit Wasser 2: diluted NaOH-solution / Schuetteln mit Luft und nachfolgenden Ansaeuern View Scheme |
-
-
482-89-3
1H,1H'-2,2'-Biindolylidene-3,3'-dione

-
A
-
6374-91-0
5,7-Dibromoisatin

-
B
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With bromine |
-
-
92635-29-5
3,3,5-tribromo-2-oxindole

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With methanol |

| Conditions | Yield |
|---|---|
| nachfolgendes Ansaeuern; |
-
-
861527-50-6
5-bromo-benz[c]isoxazole-3-carboxylic acid

-
-
7664-41-7
ammonia

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| Ansaeuern; |


-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| bei der Nitrierung; |
-
-
7664-93-9
sulfuric acid

-
-
482-89-3
1H,1H'-2,2'-Biindolylidene-3,3'-dione

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
7732-18-5
water

-
-
7726-95-6
bromine

-
-
482-89-3
1H,1H'-2,2'-Biindolylidene-3,3'-dione

-
A
-
6374-91-0
5,7-Dibromoisatin

-
B
-
87-48-9
5-Bromo-1H-indole-2,3-dione


-
A
-
861527-50-6
5-bromo-benz[c]isoxazole-3-carboxylic acid

-
B
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With water Behandeln des Niederschlags mit verd. Natronlauge; | |
| With hydrogenchloride |
-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
13278-67-6
4-(4-methylphenyl)-3-thiosemicarbazide

-
-
92461-01-3
C16H13BrN4OS

| Conditions | Yield |
|---|---|
| In ethanol for 4h; Heating; | 100% |
| With K10 clay In water for 0.1h; Temperature; Time; Microwave irradiation; Green chemistry; | 86% |
-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
17852-52-7, 27918-19-0
4-hydrazinobenzene-1-sulfonamide hydrochloride

| Conditions | Yield |
|---|---|
| In ethanol for 2h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-Bromo-1H-indole-2,3-dione With sodium hydroxide In water at 50℃; for 0.333333h; Stage #2: With sulfuric acid; sodium nitrite In water at 0℃; for 1h; Stage #3: With hydrogenchloride; tin(ll) chloride In water at 20℃; for 16h; | 100% |
| Stage #1: 5-Bromo-1H-indole-2,3-dione With sodium hydroxide In water for 1h; Stage #2: With sulfuric acid; sodium nitrite In water at 0℃; Stage #3: With hydrogenchloride; tin(ll) chloride In water at 20℃; for 5h; | 30% |
| Stage #1: 5-Bromo-1H-indole-2,3-dione With sodium hydroxide at 20 - 50℃; for 1.5h; Stage #2: With sulfuric acid; sodium nitrite In water at 0℃; for 0.75h; Stage #3: With hydrogenchloride; tin(ll) chloride In water at 0℃; for 1.16667h; |
-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
762-04-9
phosphonic acid diethyl ester

-
-
109-77-3
malononitrile

-
-
1210948-58-5
diethyl 5-bromo-3-(dicyanomethyl)-2-oxoindolin-3-ylphosphonate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 0.333333h; Ionic liquid; | 100% |
| With ZnO nano-rods at 20℃; for 0.916667h; Neat (no solvent); | 88% |
| at 50℃; for 1.5h; neat (no solvent); | 83% |


| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 110℃; for 16h; | 100% |
-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
74-88-4
methyl iodide

-
-
2058-72-2
5-bromo-1-methyl-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 20℃; for 12h; | 99% |
| With caesium carbonate In acetonitrile at 20℃; for 7h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 87% |
-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
107-21-1
ethylene glycol

-
-
75822-54-7
5’-bromo-1‘,2’-dihydrospiro[1,3-dioxolane-2,3’-indole]-2’-one

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 5h; Reflux; | 99% |
| With toluene-4-sulfonic acid In toluene for 5h; Reflux; | 99% |
| With toluene-4-sulfonic acid In toluene at 25 - 110℃; for 4h; Inert atmosphere; | 91% |
-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
109-77-3
malononitrile

-
-
130817-33-3
2-(5-bromo-2-oxoindolin-3-ylidene)malononitrile

| Conditions | Yield |
|---|---|
| With 1,3,5-triazine-piperazine immobilised mesoporous silica In tetrahydrofuran at 20℃; for 0.25h; | 99% |
| In water at 20℃; for 0.166667h; Knoevenagel Condensation; Green chemistry; | 99% |
| With water at 20℃; for 0.25h; Knoevenagel condensation; Neat (no solvent); | 96% |
-
-
120-72-9
indole

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
1242341-39-4
(S)-5-bromo-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one

| Conditions | Yield |
|---|---|
| With 9-O-benzyl-6'-hydroxycinchonidine In tetrahydrofuran at 20℃; for 96h; Friedel Crafts type reaction; Molecular sieve; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
1006-94-6
5-methoxylindole

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
1242341-44-1
(S)-5-bromo-3-hydroxy-3-(5-methoxy-1H-indol-3-yl)indolin-2-one

| Conditions | Yield |
|---|---|
| With 9-O-benzyl-6'-hydroxycinchonidine In tetrahydrofuran at 20℃; for 96h; Friedel Crafts type reaction; Molecular sieve; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
79878-27-6
1-ethyl-7-methyl-4-oxo-1, 4-dihydro-1,8-naphthyridine-3-carbohydrazide

-
-
1253047-30-1
N'-(5-bromo-2-oxoindolin-3-ylidene)-1-ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carbohydrazide

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol Reflux; | 99% |
-
-
1533-03-5
3-(trifluoromethyl)propiophenone

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
1586-30-7
6-bromo-3-methyl-2-(3-trifluoromethyl-phenyl)-quinoline-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-(trifluoromethyl)propiophenone; 5-Bromo-1H-indole-2,3-dione With potassium hydroxide In ethanol; water at 85℃; for 3h; Stage #2: With hydrogenchloride In water at 0℃; pH=~ 3; Product distribution / selectivity; | 99% |
| Stage #1: 3-(trifluoromethyl)propiophenone; 5-Bromo-1H-indole-2,3-dione With potassium hydroxide In ethanol; water for 1h; Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=3; Product distribution / selectivity; | 93% |

| Conditions | Yield |
|---|---|
| With water at 20℃; for 24h; | 99% |
-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
1896-62-4
(E)-benzalacetone

-
-
1609386-02-8
(E)-5-bromo-3-hydroxy-3-(2-oxo-4-phenylbut-3-en-1-yl)indolin-2-one

| Conditions | Yield |
|---|---|
| With L-arginine In methanol at 25℃; for 48h; Aldol Addition; | 99% |
| With L-arginine In methanol at 25℃; for 48h; |
-
-
4149-06-8
3-amino-1-phenyl-2-pyrazolin-5-one

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
83-33-0
inden-1-one

| Conditions | Yield |
|---|---|
| With acetic acid In water at 90℃; for 5h; Green chemistry; | 99% |

-
-
50-00-0
formaldehyd

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
79-19-6
thiosemicarbazide

-
-
59052-85-6
2-(piperazin-1-ylmethyl)-1H-benzimidazole

| Conditions | Yield |
|---|---|
| With sulfonic acid immobilized on metformin-p-formylbenzoic acid-based schiff base-coated Fe3O4 nanocatalyst In ethanol for 2h; Reflux; | 99% |

-
-
50-00-0
formaldehyd

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
79-19-6
thiosemicarbazide

-
-
55686-91-4
2-(1-piperazinyl)-quinoxaline

| Conditions | Yield |
|---|---|
| With sulfonic acid immobilized on metformin-p-formylbenzoic acid-based schiff base-coated Fe3O4 nanocatalyst In ethanol for 2h; Reflux; | 99% |


| Conditions | Yield |
|---|---|
| With formaldehyd In ethanol; water for 3h; Reflux; | 99% |
-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
79-19-6
thiosemicarbazide

-
-
4553-11-1
2-(5-bromo-2,3-dihydro-2-oxo-1H-indol-3-ylidene)hydrazinecarbothioamide

| Conditions | Yield |
|---|---|
| With sulfonic acid immobilized on metformin-p-formylbenzoic acid-based schiff base-coated Fe3O4 nanocatalyst In ethanol Reflux; | 98% |
| With montmorillonite K10 acid washed clay (H0=-6 to -8) for 0.0666667h; Microwave irradiation; Neat (no solvent); | 92% |
| In acetic acid for 0.5h; Heating; | 84% |

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 2h; | 98% |
| With acetic acid; 3-chloro-benzenecarboperoxoic acid at 20℃; for 4h; | 87% |
| With sulfuric acid; dihydrogen peroxide In water; acetic acid at 60 - 70℃; for 2h; | 83% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; for 48h; Temperature; | 98% |
| Stage #1: 5-Bromo-1H-indole-2,3-dione With potassium carbonate In acetonitrile at 20℃; for 0.5h; Stage #2: benzyl chloride In acetonitrile at 80℃; for 3h; | 92% |
| With potassium hydroxide In ethanol at 60℃; for 1.5h; | 69% |
| With potassium carbonate In N,N-dimethyl-formamide Reflux; |
-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

| Conditions | Yield |
|---|---|
| In 1,4-dioxane Heating; | 98% |
-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
A
-
791-28-6
Triphenylphosphine oxide

| Conditions | Yield |
|---|---|
| for 3h; Wittig reaction; Heating; | A n/a B 98% |


| Conditions | Yield |
|---|---|
| In water at 70℃; for 0.166667h; | 98% |
| With titanium(IV) oxide In water at 20℃; Green chemistry; | 98% |
| With Fe3O4(at)SiO2(at)Bi2O3 nanoparticles In water at 80℃; for 0.5h; | 97% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 24h; pH=5 - 6; Heating; | 98% |
-
-
89335-21-7
2-{[5-(4-methoxyphenyl)-1,3,5-oxadiazol-2-yl]imino}-4-thiazolidinone

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic anhydride In acetic acid at 150 - 160℃; for 5h; | 98% |

| Conditions | Yield |
|---|---|
| With silica-bonded 5-n-propyl-octahydro-pyrimido[1,2-a]azepinium chloride In ethanol at 20℃; for 2.5h; | 98% |
| With mesoporous silica SBA-15 supported 1,4-diazabicyclo[2.2.2]octane In water at 50℃; for 0.05h; Temperature; Time; | 97% |
| With caspian isinglass In water at 60℃; for 0.0833333h; | 97% |

-
-
95-20-5
2-methyl-1H-indole

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
695159-60-5
5-bromo-3,3-bis(2-methyl-1H-indol-3-yl)indolin-2-one

| Conditions | Yield |
|---|---|
| With carboxylic acid supported on ferrite-silica nanoparticle In water at 80℃; for 0.5h; Green chemistry; | 98% |
| With sulfuric acid-functionalized magnetite nanoparticles In acetonitrile at 20℃; for 1h; Sonication; | 96% |
| With Fe3O4(at)SiO2(at)Bi2O3 nanoparticles In water at 80℃; for 0.416667h; | 96% |

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonim bromide In water at 80℃; for 0.5h; Green chemistry; | 98% |
| With scandium tris(dodecyl sulfate) In water at 80℃; for 0.5h; Green chemistry; | 96% |
| With β‐cyclodextrin In water at 60℃; for 1.5h; | 95% |
-
-
603-76-9
1-methylindole

-
-
87-48-9
5-Bromo-1H-indole-2,3-dione

-
-
695158-31-7
5-bromo-3,3-bis(1-methyl-1H-indol-3-yl)indolin-2-one

| Conditions | Yield |
|---|---|
| With silica In neat (no solvent) at 20℃; for 0.0166667h; Green chemistry; | 98% |
| With H6P2W18O62 In water at 60℃; for 0.5h; | 92% |
| With 1,4-diazaniumbicyclo[2.2.2]octane diacetate for 0.166667h; | 92% |
5-Bromoisatin Specification
The 5-Bromoindole-2,3-dione with CAS registry number of 87-48-9 is also known as 5-Bromoisatin. The IUPAC name is 5-Bromo-1H-indole-2,3-dione. It belongs to product categories of Indole/indoline/oxindole; Indole and Indoline; Indane/Indanone and Derivatives; Indoles; Simple Indoles; Halogenated Heterocycles; Heterocyclic Building Blocks; IndolesBuilding Blocks. Its EINECS registry number is 201-747-7. In addition, the formula is C8H4BrNO2 and the molecular weight is 226.03. This chemical is a orange crystalline powder and should be sealed in cool, dry place.
Physical properties about 5-Bromoindole-2,3-dione are: (1)ACD/LogP: 1.33; (2)ACD/LogD (pH 5.5): 1.33; (3)ACD/LogD (pH 7.4): 1.31; (4)ACD/BCF (pH 5.5): 6.04; (5)ACD/BCF (pH 7.4): 5.78; (6)ACD/KOC (pH 5.5): 126.08; (7)ACD/KOC (pH 7.4): 120.73; (8)#H bond acceptors: 3; (9)#H bond donors: 1; (10)Index of Refraction: 1.649; (11)Molar Refractivity: 45.11 cm3; (12)Molar Volume: 123.7 cm3; (13)Surface Tension: 58.4 dyne/cm; (14)Density: 1.826 g/cm3.
Preparation of 5-Bromoindole-2,3-dione: it is prepared by reaction of indole-2,3-dione. The reaction needs reagents N-bromosaccharin, SiO2 and solvent CH2Cl2 at the temperature of 20 °C for 12 hours. The yield is about 58%.

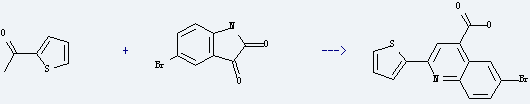
Uses of 5-Bromoindole-2,3-dione: it is used to produce 6-bromo-2-thiophen-2-yl-quinoline-4-carboxylic acid by reaction with 1-thiophen-2-yl-ethanone. The reaction occurs with solvents NaOH, water and other condition of heating for 2 hours. The yield is about 76%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to respiratory system and skin. What's more, it has risk of serious damage to eyes. During using it, wear suitable protective clothing, gloves and eye/face protection. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC2=C(C=C1Br)C(=O)C(=O)N2
2. InChI: InChI=1S/C8H4BrNO2/c9-4-1-2-6-5(3-4)7(11)8(12)10-6/h1-3H,(H,10,11,12)
3. InChIKey: MBVCESWADCIXJN-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 437mg/kg (437mg/kg) | LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | Pharmaceutical Chemistry Journal Vol. 15, Pg. 858, 1981. |
| mouse | LD50 | oral | 3430mg/kg (3430mg/kg) | BEHAVIORAL: EXCITEMENT BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 53(2), Pg. 93, 1988. |
| rat | LD50 | intraperitoneal | 680mg/kg (680mg/kg) | BEHAVIORAL: EXCITEMENT BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 53(2), Pg. 93, 1988. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View