-
Name
5-Methyl-2-hexanone
- EINECS 203-737-8
- CAS No. 110-12-3
- Article Data73
- CAS DataBase
- Density 0.806 g/cm3
- Solubility 5.4 g/L (20 °C) in water
- Melting Point -74 °C
- Formula C7H14O
- Boiling Point 144 °C at 760 mmHg
- Molecular Weight 114.188
- Flash Point 41.1 °C
- Transport Information UN 2302 3/PG 3
- Appearance clear colourless liquid
- Safety 23-24/25
- Risk Codes 10-20
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 2-Methyl-5-hexanone;3-Methylbutyl methyl ketone;Isoamyl methyl ketone;Isopentyl methyl ketone;MIAK;Methyl isoamyl ketone;Methyl isopentyl ketone;
- PSA 17.07000
- LogP 2.01160
Synthetic route
-
-
1669-43-8, 5166-53-0, 1821-29-0
(E)-4-isopropylbut-3-en-2-one

-
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With water; zinc; chloro(1,5-cyclooctadiene)rhodium(I) dimer In 1,4-dioxane at 90℃; for 20h; | 99% |
| With dicobalt octacarbonyl; water In 1,2-dimethoxyethane for 2h; Heating; | 91 % Chromat. |
| With 1,3-bis-(diphenylphosphino)propane; caesium carbonate; isopropyl alcohol; bis(1,5-cyclooctadiene)diiridium(I) dichloride In toluene at 80℃; for 4h; |
-
-
73503-97-6
5-methyl-2-(trimethylsiloxy)-1-hexene

-
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With tributyltin fluoride; dichlorobis(tri-O-tolylphosphine)palladium In benzene for 0.5h; Product distribution; Heating; other catalysts; | 96% |

| Conditions | Yield |
|---|---|
| With pyridine; tert-butylhypochlorite In dichloromethane for 1h; | 93% |
| With Oxone; sodium chloride In water; ethyl acetate at 20℃; for 4h; | 80% |
| With potassium permanganate; sulfuric acid |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium chlorite In water at 20℃; for 0.0833333h; | 92% |

-
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With sulfuric acid In benzene for 4h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water; acetonitrile at 55℃; for 12h; Wacker Oxidation; | 84% |
-
-
876-27-7
4-chlorophenyl acetate

-
-
146513-95-3, 146560-03-4, 93399-99-6
(rac,E)-5-methylhex-3-en-2-ol

-
A
-
1669-43-8, 5166-53-0, 1821-29-0
(E)-4-isopropylbut-3-en-2-one

-
B
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With RuCl3H(p-cymene)2; immobilized lipase from Pseudomonas cepacia; triethylamine In dichloromethane at 20 - 25℃; for 48h; Acetylation; oxidation; reduction; Enzymatic reaction; | A n/a B n/a C 83% |

-
-
67-56-1
methanol

-
-
75-30-9
2-iodo-propane

-
-
97-94-9
triethyl borane

-
-
78-94-4
methyl vinyl ketone

-
A
-
591-78-6
n-hexan-2-one

-
B
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| In hexane; benzene at 25℃; for 0.0833333h; | A 15% B 79% |

-
-
67-56-1
methanol

-
-
75-30-9
2-iodo-propane

-
-
78-94-4
methyl vinyl ketone

-
A
-
591-78-6
n-hexan-2-one

-
B
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With triethyl borane In hexane; benzene at 25℃; for 0.0833333h; | A 15% B 79% |
| With triethyl borane In hexane; benzene | A 15% B 79% |

-
-
67-56-1
methanol

-
-
97-94-9
triethyl borane

-
-
78-94-4
methyl vinyl ketone

-
A
-
591-78-6
n-hexan-2-one

-
B
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With 2-iodo-propane In hexane; benzene | A 15% B 79% |

-
-
108-86-1
bromobenzene

-
-
73503-97-6
5-methyl-2-(trimethylsiloxy)-1-hexene

-
A
-
27993-43-7
5-methyl-1-phenylhexan-2-one

-
B
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With dichlorobis(tri-O-tolylphosphine)palladium; tributyltin fluoride In benzene for 4h; Heating; | A 62% B 29% |

-
-
187727-38-4
5-methyl-2,3-hexanediol

-
A
-
27944-79-2
2,4-dimethylpentanal

-
B
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With poly(pyrrole) In n-heptane Reflux; Inert atmosphere; | A 59% B 29% |

| Conditions | Yield |
|---|---|
| (tetramethylethylenediamine)Zn(O-t-Bu)Cl In tetrahydrofuran; diethyl ether at 0℃; for 0.5h; Yields of byproduct given; | A n/a B 51% |

-
-
13706-86-0
5-methyl-2,3-hexanedione

-
A
-
623-56-3
5-methyl-3-hexanone

-
B
-
623-55-2
(+-)-5-methyl-3-hexanol

-
C
-
627-59-8
5-methylhexan-2-ol

-
D
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With samarium diiodide; water In tetrahydrofuran at 20℃; for 0.15h; | A 15% B 51% C 21% D 5% |


| Conditions | Yield |
|---|---|
| In benzene at 35 - 50℃; for 16h; | 13% |

| Conditions | Yield |
|---|---|
| With ethanol; palladium Hydrogenation; | |
| With Ir(I)[PPh2CH2CH2Si(OEt)3]3Cl; caesium carbonate In propan-1-ol at 80℃; for 4h; Reagent/catalyst; chemoselective reaction; | |
| With hydrogen In water at 20℃; under 750.075 Torr; Reagent/catalyst; Schlenk technique; Green chemistry; | |
| With methanol; C25H29ClNO2Rh; potassium carbonate at 90℃; for 1h; chemoselective reaction; | 88 %Chromat. |

| Conditions | Yield |
|---|---|
| With potassium permanganate; acetone |
-
-
66256-45-9
2,3,6-trimethyl-heptan-2-ol

-
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With chromic acid |

| Conditions | Yield |
|---|---|
| With barium dihydroxide | |
| With potassium hydroxide | |
| With sodium hydroxide | |
| With sodium hydroxide In ethanol |

| Conditions | Yield |
|---|---|
| With diethyl ether Nicht einheitliches!Es liefert ein Oxim vom Kp:196grad und ein Semicarbazon vom F:141grad; |

| Conditions | Yield |
|---|---|
| With thorium dioxide |

| Conditions | Yield |
|---|---|
| (i) Li, liq. NH3, (ii) aq. Na2Cr2O7, aq. H2SO4; Multistep reaction; |
-
-
56161-54-7
2-methyl-2-(2-(phenylsulfonyl)ethyl)-1,3-dioxolane

-
-
75-26-3
isopropyl bromide

-
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| Multistep reaction; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; camphor-10-sulfonic acid; dihydrogen peroxide; 9-bora-bicyclo[3.3.1]nonane In tetrahydrofuran at 0℃; for 2h; Product distribution; various hydroborating agents; |

| Conditions | Yield |
|---|---|
| With hydrogen | |
| With hydrogen In propan-1-ol at 25℃; Thermodynamic data; enthalpy of hydrogenation; |
-
-
3240-09-3
5-methyl-5-hexen-2-one

-
A
-
50551-88-7
5-methylhex-5-en-2-ol

-
B
-
627-59-8
5-methylhexan-2-ol

-
C
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With hydrogen; carbon monoxide In cyclohexane at 20 - 25℃; under 760 - 798 Torr; for 1h; Product distribution; other catalyst; | A 8 % Chromat. B 42 % Chromat. C 50 % Chromat. |
| With trans-RuCl(6,6'-dihydroxyterpyridine)(PPh3)2PF6; potassium tert-butylate; isopropyl alcohol at 80℃; for 24h; Inert atmosphere; Sealed tube; chemoselective reaction; | A 66 %Chromat. B n/a C n/a |

-
-
41595-17-9
2,2-Bis-tert-butylperoxy-5-methyl-hexane

-
A
-
75-91-2
tert.-butylhydroperoxide

-
B
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| With sulfuric acid; water In 1,4-dioxane at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| Stage #1: 5-Methyl-2-hexanone; N-phenylphenylene-1,4-diamine at 120 - 125℃; for 1.5h; Stage #2: With palladium on activated charcoal; hydrogen at 155 - 163℃; under 15001.5 Torr; for 1h; Reagent/catalyst; Pressure; | 99.79% |
| With hydrogen at 160℃; under 22502.3 Torr; Temperature; Pressure; | |
| With hydrogen under 22502.3 Torr; for 3h; Catalytic behavior; Reagent/catalyst; Pressure; Autoclave; Heating; |

| Conditions | Yield |
|---|---|
| Stage #1: 4-ntrophenyl(phenyl)amine; 5-Methyl-2-hexanone at 120 - 125℃; for 1h; Stage #2: With palladium on activated charcoal; hydrogen at 155 - 163℃; under 7500.75 Torr; for 1h; Reagent/catalyst; Pressure; | 99.3% |
-
-
59-88-1
phenylhydrazine hydrochloride

-
-
110-12-3
5-Methyl-2-hexanone

-
-
1310681-93-6
3-isobutyl-2-methyl-1H-indole

| Conditions | Yield |
|---|---|
| With 1,3,5-trichloro-2,4,6-triazine In ethanol at 80℃; for 2h; Fischer Indole Synthesis; | 98% |
| With 4-methylbenzenesulfonic acid-based ionic liquid supported on silica gel In ethanol at 20℃; for 4h; Fischer Indole Synthesis; | 91% |
-
-
120-80-9
benzene-1,2-diol

-
-
110-12-3
5-Methyl-2-hexanone

-
-
30122-03-3
2-methyl-2-(3-methylbut-1-yl)-1,3-benzodioxole

| Conditions | Yield |
|---|---|
| p-toluenesulfonic acid monohydrate In toluene | 97% |
| With toluene-4-sulfonic acid In toluene for 24h; Heating; | 79% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-Methyl-2-hexanone With (R,R)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminoaluminum(III) chloride; (tert-Butoxycarbonylmethylene)triphenylphosphorane; Triphenylphosphine oxide In dichloromethane at -40℃; for 0.5h; Inert atmosphere; Stage #2: trimethylsilyl cyanide In dichloromethane at -40℃; for 36h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-Methyl-2-hexanone With (R,R)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminoaluminum(III) chloride; ethyl (triphenylphosphoranylidene)acetate In diethyl ether at -30℃; for 0.5h; Schlenk technique; Inert atmosphere; Stage #2: C4H8ClNSi In diethyl ether at -30℃; for 6h; Solvent; Schlenk technique; Inert atmosphere; enantioselective reaction; | 97% |
-
-
110-12-3
5-Methyl-2-hexanone

-
-
71700-44-2
3-bromo-5-methylhexan-2-one

| Conditions | Yield |
|---|---|
| 96% | |
| With trimethylsilyl bromide; dimethyl sulfoxide In acetonitrile for 2h; Ambient temperature; |
-
-
118427-29-5
(4-isopropylphenyl)hydrazine hydrochloride

-
-
110-12-3
5-Methyl-2-hexanone

-
-
1456718-37-8
3-isobutyl-5-isopropyl-2-methyl-1H-indole

| Conditions | Yield |
|---|---|
| With 1,3,5-trichloro-2,4,6-triazine In ethanol at 80℃; for 2h; Fischer Indole Synthesis; | 96% |
-
-
110-12-3
5-Methyl-2-hexanone


| Conditions | Yield |
|---|---|
| at 130℃; for 48h; | 95% |

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate; bis(1,5-cyclooctadiene)diiridium(I) dichloride; 5-(dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-yl)-10,11-dihydro-5H-dibenzo[b,f]azepine; hydrogen; trifluoroacetic acid In dichloromethane at 30℃; under 45603.1 Torr; for 13h; Inert atmosphere; Glovebox; Molecular sieve; Autoclave; enantioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: ortho-tolylmagnesium bromide; 5-Methyl-2-hexanone In tetrahydrofuran; diethyl ether at -78 - 25℃; for 3h; Inert atmosphere; Sealed tube; Stage #2: With water; ammonium chloride | 95% |

| Conditions | Yield |
|---|---|
| In toluene for 24h; Alkylation; | 94% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; 1,1,3,3-Tetramethyldisiloxane; 1,3-bis(4-cyano-3,5-bis(trifluoromethyl)phenyl)thiourea In 1,4-dioxane; dichloromethane at 20℃; for 0.5h; Molecular sieve; | 94% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 20℃; for 4h; | 92% |
| With sodium tetrahydroborate In methanol at 0 - 23℃; for 6h; | 76% |
| With hydrogenchloride; isopropyl alcohol; nickel for 1.33333h; Heating; | 61% |
| With sodium amalgam |

| Conditions | Yield |
|---|---|
| Stage #1: 5-Methyl-2-hexanone With pyridine; hydroxylamine hydrochloride In methanol at 20℃; for 6h; Stage #2: 4-trifluoromethyl-phenyl acetyl chloride With triethylamine In dichloromethane at 0℃; for 0.166667h; | 92% |

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In tetrahydrofuran; toluene 1.) 20 deg C, 90 min, 2.) 20 deg C, 3 h; | 91% |

| Conditions | Yield |
|---|---|
| With poly(ethylene)-supported activated zinc In tetrahydrofuran at 20℃; for 2h; | 91% |
-
-
110-12-3
5-Methyl-2-hexanone

-
-
31039-14-2
2-methyl-5-((5-methylhexan-2-yl)oxy)hexane

| Conditions | Yield |
|---|---|
| With triethylsilane; trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0 - 20℃; for 2h; | 91% |
-
-
123-11-5
4-methoxy-benzaldehyde

-
-
110-12-3
5-Methyl-2-hexanone

-
-
82297-69-6
1-(4-methoxyphenyl)-6-methylhept-1-en-3-one

| Conditions | Yield |
|---|---|
| barium dihydroxide In ethanol for 1h; Heating; | 89% |

| Conditions | Yield |
|---|---|
| With nickel(0) nanoparticles In tetrahydrofuran at 76℃; for 20h; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide; toluene-4-sulfonic acid In toluene at 20℃; for 1.5h; | 86% |
-
-
480-96-6
benzofurazan oxide

-
-
110-12-3
5-Methyl-2-hexanone

-
-
77236-80-7
2-Isobutyl-3-methylchinoxalin-1,4-dioxid

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 40 - 50℃; | 85% |
-
-
6336-44-3, 7547-96-8, 7547-97-9, 88982-39-2
allylboronic acid

-
-
110-12-3
5-Methyl-2-hexanone

| Conditions | Yield |
|---|---|
| Stage #1: 5-Methyl-2-hexanone With ammonia In methanol at 20℃; for 0.25h; Stage #2: allylboronic acid In methanol at 20℃; for 16h; | 85% |

| Conditions | Yield |
|---|---|
| With copper(II) oxide In acetonitrile for 13h; Heating; | 84% |
5-Methyl-2-hexanone Consensus Reports
Reported in EPA TSCA Inventory.
5-Methyl-2-hexanone Standards and Recommendations
OSHA PEL: TWA 50 ppm
ACGIH TLV: TWA 50 ppm
NIOSH REL: Ketones (Methyl Isoamyl Ketone) TWA 230 mg/m3
DOT Classification: 3; Label: Flammable Liquid
5-Methyl-2-hexanone Specification
The IUPAC name of Methyl isoamyl ketone is 5-methylhexan-2-one. With the CAS registry number 110-12-3 and EINECS 203-737-8, it is also named as 2-Hexanone, 5-methyl-. The classification codes are Agricultural Chemical; Skin / Eye Irritant; TSCA Flag T [Subject to the Section 4 test rule under TSCA]; Unspecified / Unclassified pesticide. It is clear colourless liquid with a pleasant fruity odor. When heated to decomposition it emits acrid smoke and irritating fumes. So the storage environment should be well-ventilated, low-temperature and dry. Keep Methyl isoamyl ketone separate from oxidant.
The other characteristics of this product can be summarized as: (1)ACD/LogP: 1.78; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.78; (4)ACD/LogD (pH 7.4): 1.78; (5)ACD/BCF (pH 5.5): 13.36; (6)ACD/BCF (pH 7.4): 13.36; (7)ACD/KOC (pH 5.5): 222.62; (8)ACD/KOC (pH 7.4): 222.62; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.401; (14)Molar Refractivity: 34.46 cm3; (15)Molar Volume: 141.5 cm3; (16)Polarizability: 13.66×10-24 cm3; (17)Surface Tension: 23.9 dyne/cm; (18)Density: 0.806 g/cm3; (19)Flash Point: 41.1 °C; (20)Enthalpy of Vaporization: 38.11 kJ/mol; (21)Boiling Point: 144 °C at 760 mmHg; (22)Vapour Pressure: 5.2 mmHg at 25°C.
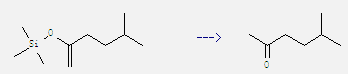
Preparation of Methyl isoamyl ketone: It can be obtained by 5-methyl-2-(trimethylsiloxy)-1-hexene. This reaction needs reagent tributyltin fluoride, catalytic agent PdCl2(P(o-MeC6H4)3)2 and solvent benzene by heating. The reaction time is 30 min. The yield is 96%.
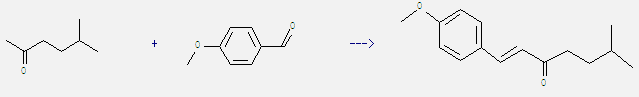
Uses of Methyl isoamyl ketone: It is used in organic synthesis. For example: it can react with 4-methoxy-benzaldehyde to get 1-(4-methoxy-phenyl)-6-methyl-hept-1-en-3-one. This reaction needs catalytic agent Ba(OH)2 C-200 and solvent ethanol by heating. The reaction time is 1 hours. The yield is 89%.
When you are using this chemical, please be cautious about it as the following:
It is not only flammable, but also harmful by inhalation. So people should not breathe vapour and avoid contact with skin and eyes.
People can use the following data to convert to the molecule structure.
1. SMILES:O=C(C)CCC(C)C
2. InChI:InChI=1/C7H14O/c1-6(2)4-5-7(3)8/h6H,4-5H2,1-3H3
3. InChIKey:FFWSICBKRCICMR-UHFFFAOYAQ
The following are the toxicity data which has been tested.
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD | skin | > 20mL/kg (20mL/kg) | CARDIAC: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | National Technical Information Service. Vol. OTS0535379, |
| mouse | LD50 | intraperitoneal | 800mg/kg (800mg/kg) | CARDIAC: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | National Technical Information Service. Vol. OTS0535379, |
| mouse | LD50 | oral | 2542mg/kg (2542mg/kg) | Toxicology Letters. Vol. 30, Pg. 13, 1986. | |
| rabbit | LD50 | skin | 10mL/kg (10mL/kg) | Union Carbide Data Sheet. Vol. 8/7/1963, | |
| rat | LC50 | inhalation | 3813ppm/6H (3813ppm) | CARDIAC: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | National Technical Information Service. Vol. OTS0535379, |
| rat | LD50 | oral | 3200mg/kg (3200mg/kg) | CARDIAC: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | National Technical Information Service. Vol. OTS0535379, |
| rat | LDLo | intraperitoneal | 400mg/kg (400mg/kg) | CARDIAC: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | National Technical Information Service. Vol. OTS0535379, |
Related Products
- 5-Methyl-2-hexanone
- 110123-09-6
- 110130-88-6
- 110-13-4
- 11013-97-1
- 1101-39-9
- 110140-69-7
- 1101-41-3
- 110143-52-7
- 110143-57-2
- 110-14-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View