-
Name
4-Acetamidophenol
- EINECS 203-157-5
- CAS No. 103-90-2
- Article Data344
- CAS DataBase
- Density 1.249 g/cm3
- Solubility 14 g/L (20 °C) in water
- Melting Point 168-172 °C(lit.)
- Formula C8H9NO2
- Boiling Point 387.8 °C at 760 mmHg
- Molecular Weight 151.165
- Flash Point 188.4 °C
- Transport Information
- Appearance White crystalline powder
- Safety 26-36-61-37/39-22
- Risk Codes 22-36/37/38-52/53-36/38-40
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Acetamide,N-(4-hydroxyphenyl)-;Acetanilide,4'-hydroxy- (7CI,8CI);4-(Acetylamino)phenol;4-(N-Acetylamino)phenol;4-Acetamidophenol;4-Acetaminophenol;4-Hydroxyacetanilide;4'-Hydroxyacetanilide;Alpiny;Alvedon;Anhiba;Apamid;Apamide;Banesin;Ben-u-ron;Bickie-mol;Biocetamol;Cetadol;Clixodyne;Daphalgan;Datril;Dirox;Enelfa;Eu-Med;Exdol;Gattaphen T;Homoolan;Jin Gang;Korum;Lestemp;Liquagesic;Lonarid;Lyteca;Lyteca Syrup;Minoset;Minoset Plus;Momentum;Multin;N-(4-Hydroxyphenyl)acetamide;N-Acetyl-4-aminophenol;N-Acetyl-4-hydroxyaniline;Pacemo;Pacemol;Panadol;Panadol Actifast;Panadol Extend;Panaleve;Panasorb;Panets;Panodil;Paracetamol;Paracetamol DC;Paracetamole;Parageniol;Paralen;Paramol;Paraspen;Parelan;Parmol;Pasolind N;Pedric;k*e*t*a*m*i*n*e ,kush available;
- PSA 49.33000
- LogP 1.42360
Synthetic route

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride In acetonitrile at 110℃; for 1h; Solvent; Time; chemoselective reaction; | 100% |
| With hydroxylamine hydrochloride; tetrachlorosilane at 160℃; for 0.0583333h; microwave irradiation; | 92% |
| With mesitylenesulfonylhydroxylamine In acetonitrile at 20℃; for 6h; | 92% |

| Conditions | Yield |
|---|---|
| With sodium dodecyl-sulfate In water | 99% |
| With silica gel for 0.5h; Time; Milling; | 99% |
| With sulfuric acid supported on poly(4-vinylpyridine) (P4VP) In dichloromethane at 20℃; for 1.25h; | 98% |

| Conditions | Yield |
|---|---|
| With ammonium acetate In methanol at 20℃; for 4.5h; | 99% |
| With ytterbium(III) triflate In isopropyl alcohol for 15h; Deacetylation; Heating; | 96% |
| With sodium perborate In methanol at 25℃; for 0.5h; | 90% |
-
-
34523-34-7
4-hydroxyacetophenone oxime

-
-
103-90-2
4-acetaminophenol

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; perfluoropinacol; 2-methoxycarbonylphenylboronic acid In nitromethane at 20℃; for 24h; Beckmann Rearrangement; chemoselective reaction; | 99% |
| With 2,2-dichloro-1,3-dicyclohexylimidazolidine-4,5-dione In acetonitrile at 80℃; for 0.333333h; Inert atmosphere; Schlenk technique; Green chemistry; | 98% |
| With carbon tetrabromide; Eosin Y; N,N-dimethyl-formamide In acetonitrile at 20℃; for 14h; Beckmann Rearrangement; Irradiation; Inert atmosphere; Green chemistry; | 96% |

| Conditions | Yield |
|---|---|
| at 80 - 130℃; Temperature; | 98% |
| With sodium sulfite for 16h; Reflux; | 83% |
| With Methyl formate; dodecacarbonyl-triangulo-triruthenium at 180℃; for 8h; | 92 % Chromat. |
| With hydrogen at 100℃; under 760.051 Torr; for 24h; Sealed tube; | 82 %Chromat. |
| With platinum; hydrogen at 100℃; for 24h; |

| Conditions | Yield |
|---|---|
| at 80 - 130℃; | 98% |

| Conditions | Yield |
|---|---|
| With acetic acid at 80℃; for 1h; Temperature; | 97.13% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water at 25℃; for 8h; Green chemistry; | 97% |
-
-
101251-09-6
(4-acetylaminophenyl)boronic acid

-
-
103-90-2
4-acetaminophenol

| Conditions | Yield |
|---|---|
| With water; dihydrogen peroxide In ethanol at 20℃; for 0.0166667h; Green chemistry; | 97% |
| With Oxone; potassium phosphate; 2-(biphenyl-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane In water at 70℃; for 1h; chemoselective reaction; |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate In water at 100℃; for 0.166667h; Microwave irradiation; Green chemistry; | 96% |
| at 150℃; for 72h; Inert atmosphere; Sealed tube; Green chemistry; | 96% |
| With air at 150℃; for 72h; Sealed tube; | 96% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; chloro-trimethyl-silane In methanol; water at 20℃; for 0.333333h; Reagent/catalyst; Irradiation; | 95% |
| With hydrogen In para-xylene at 130℃; under 750.075 Torr; for 24h; chemoselective reaction; | 95% |
| With samarium; acetic acid In methanol at 20℃; for 1.5h; | 80% |
| Stage #1: 4-nitro-phenol With sodium tetrahydroborate at 20℃; for 0.5h; Green chemistry; Stage #2: acetic anhydride at 120℃; for 1h; Catalytic behavior; Green chemistry; | 78% |
| With acetic acid; platinum Hydrogenation; |
-
-
123-30-8
4-amino-phenol

-
-
107866-54-6
1-acetyl-1H-1,2,3-triazolo<4,5-b>pyridine

-
-
103-90-2
4-acetaminophenol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 1h; Ambient temperature; | 95% |
-
-
123-30-8
4-amino-phenol

-
-
155164-63-9
2-acetyl-4,5-dichloropyridazin-3(2H)-one

-
-
103-90-2
4-acetaminophenol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 17℃; for 0.2h; | 95% |
-
-
198712-64-0
(E)-1-(4-hydroxyphenyl)ethan-1-one oxime

-
-
103-90-2
4-acetaminophenol

| Conditions | Yield |
|---|---|
| With 2,2-dichloro-1,3-dicyclohexylimidazolidine-4,5-dione In acetonitrile at 80℃; for 0.333333h; Beckmann Rearrangement; Inert atmosphere; Schlenk technique; | 95% |
| With 1,1,1,3',3',3'-hexafluoro-propanol; tetrabutylammonium tetrafluoroborate; water In 1,2-dichloro-ethane at 20℃; for 0.533333h; Beckmann Rearrangement; Electrochemical reaction; | 92% |
| With cerium(III) chloride; silica gel; sodium iodide for 0.133333h; Beckmann rearrangement; microwave irradiation; | 82% |
| With 1,3,5-trichloro-2,4,6-triazine In N,N-dimethyl-formamide at 20℃; for 6h; Beckmann rearrangement; | 80% |

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at 0℃; for 1.5h; Inert atmosphere; | 95% |
| With boron tribromide In dichloromethane at 20℃; Inert atmosphere; | 68% |
| With boron tribromide In dichloromethane Cooling with ice; Inert atmosphere; | 68% |
| With 1H-imidazole; iron (III) meso-tetrakis (2,6-dichlorophenylporphyrin-β-octabromo)chloride; 3-chloro-benzenecarboperoxoic acid In isopropyl alcohol; acetonitrile at 20℃; for 24h; Reagent/catalyst; | 19.2% |
-
-
103202-04-6
N-[4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]phenyl]acetamide

-
-
103-90-2
4-acetaminophenol

| Conditions | Yield |
|---|---|
| With potassium hydrogen difluoride In methanol at 20℃; for 2h; | 95% |
| With cerium (IV) sulfate tetrahydrate In methanol at 130℃; for 0.333333h; Microwave irradiation; | 94% |
| With aluminium(III) chloride hexahydrate In methanol at 100℃; for 0.25h; Solvent; Temperature; Microwave irradiation; Sealed tube; chemoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole; nickel oxinate at 150℃; | 95% |
| With 1,2,4-Triazole; 8-quinolinol; copper(II) choride dihydrate at 150℃; | 91% |
| With Imidazole hydrochloride at 150℃; for 4h; Sealed tube; | 89% |
| With ammonium iodide at 125℃; for 17h; | 29% |

| Conditions | Yield |
|---|---|
| at 80 - 130℃; | 95% |

| Conditions | Yield |
|---|---|
| With magnesia In neat (no solvent) at 70℃; for 0.166667h; Green chemistry; chemoselective reaction; | 93% |
| at 60 - 120℃; for 11h; Product distribution / selectivity; | 91.6% |
| With Starbon-400-SO3H at 130℃; for 0.05h; Microwave irradiation; chemoselective reaction; | 89% |

| Conditions | Yield |
|---|---|
| With calcium oxide In 2-methyltetrahydrofuran at 20℃; for 4h; Green chemistry; chemoselective reaction; | 93% |
| With triethylamine In dichloromethane at 0 - 20℃; for 3.16667h; Inert atmosphere; | 83% |
| With silica gel at 20℃; for 2.5h; Green chemistry; chemoselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With dmap In water at 100℃; for 0.25h; Microwave irradiation; Green chemistry; | 93% |

| Conditions | Yield |
|---|---|
| With aluminum oxide at 200℃; under 37503.8 Torr; for 0.45h; Sonication; Green chemistry; | 93% |
| With tert.-butylnitrite; trifluorormethanesulfonic acid; water at 60℃; for 24h; | 42% |

| Conditions | Yield |
|---|---|
| With Fe3O4(at)Chit-TCT-Salen-Cu(II) In water at 20℃; for 0.0833333h; chemoselective reaction; | 92% |
| With copper(II)-grafted guanidine acetic acid-modified magnetite nanoparticles In water at 20℃; for 0.0833333h; Green chemistry; chemoselective reaction; | 91% |
| With 10-methyl-9-(2,4,6-trimethylphenyl) acridinium tetrafluoroborate In acetonitrile at 20℃; for 5h; Irradiation; | 78% |
| With 10-methyl-9-(2,4,6-trimethylphenyl) acridinium tetrafluoroborate In acetonitrile at 20℃; for 5h; Irradiation; | 78% |
| With copper(ll) sulfate pentahydrate In methanol at 20℃; for 0.0833333h; | 75% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 7h; Ambient temperature; | 90% |

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In methanol for 1h; Heating; | 90% |
| With potassium hydroxide; palladium on activated charcoal In methanol at 20℃; for 48h; | 86% |
| With potassium hydroxide In methanol at 20℃; for 48h; | 86% |
| With iodine In dimethyl sulfoxide at 130℃; for 0.5h; | 83% |
-
-
24764-93-0
N,N’-(1,2-ethanediylidene)bis-hydroxyphenylamine

-
-
108-24-7
acetic anhydride

-
A
-
131543-46-9
Glyoxal

-
B
-
103-90-2
4-acetaminophenol

| Conditions | Yield |
|---|---|
| With water; sodium dodecyl-sulfate at 25 - 30℃; for 0.166667h; | A 85% B 90% |


| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride In acetonitrile at 70℃; for 15h; | A 90% B 8% |
| With hydroxylamine hydrochloride at 70 - 110℃; Solvent; chemoselective reaction; |

| Conditions | Yield |
|---|---|
| With formic acid; boron trifluoride diethyl etherate; bis-[(trifluoroacetoxy)iodo]benzene at 20℃; for 0.5h; regioselective reaction; | 89% |
| With trifluoroacetic acid In ethanol; water at 20℃; for 3h; Electrochemical reaction; Green chemistry; regioselective reaction; | 32% |
| With 1H-imidazole; iron (III) meso-tetrakis (2,6-dichlorophenylporphyrin-β-octabromo)chloride; 3-chloro-benzenecarboperoxoic acid In isopropyl alcohol; acetonitrile at 20℃; for 24h; Reagent/catalyst; | 19.2% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 25℃; for 3h; | 88% |
-
-
50-00-0
formaldehyd

-
-
103-90-2
4-acetaminophenol

-
-
109-89-7
diethylamine

-
-
121-78-8
N-{3-[(diethylamino)methyl]-4-hydroxyphenyl}acetamide

| Conditions | Yield |
|---|---|
| In ethanol at 80℃; for 1.5h; Microwave irradiation; | 100% |
| In ethanol for 12h; Heating; | 80% |
| In ethanol at 80℃; for 1h; Microwave irradiation; | 77% |


| Conditions | Yield |
|---|---|
| With ammonium bromide; ethylenediamine at 70℃; for 5h; Reagent/catalyst; Temperature; Microwave irradiation; | 100% |
| With ammonium bromide; ethylenediamine at 70℃; for 5h; Microwave irradiation; Inert atmosphere; neat (no solvent); | 99% |
| With ammonium iodide; hydrazine hydrate at 50℃; for 12h; Inert atmosphere; Sealed tube; | 97% |
-
-
103-90-2
4-acetaminophenol

-
-
339274-36-1
S-tert-butyl cyclohexylcarbamoylthioacetate

| Conditions | Yield |
|---|---|
| With silver trifluoroacetate In tetrahydrofuran at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 65℃; for 24h; Inert atmosphere; | 100% |
| With sodium ethanolate |
-
-
103-90-2
4-acetaminophenol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
103202-04-6
N-[4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]phenyl]acetamide

| Conditions | Yield |
|---|---|
| With ferric hydrogen sulphate; triethylamine at 20℃; for 60h; Inert atmosphere; chemoselective reaction; | 100% |
| With 1H-imidazole In tetrahydrofuran for 16h; Schlenk technique; Inert atmosphere; | 97% |
| With 1H-imidazole Sealed tube; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-acetaminophenol With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 5-nitro-2-fluorotoluene In N,N-dimethyl-formamide at 140℃; for 5h; Solvent; | 99.5% |
-
-
103-90-2
4-acetaminophenol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
301337-51-9
toluene-4-sulfonic acid 4-acetylaminophenyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran; water at 0 - 20℃; for 2h; Green chemistry; | 99% |
| With triethylamine In dichloromethane for 16h; | 62% |
| With sodium carbonate | |
| With pyridine In dichloromethane at 20℃; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20 - 25℃; for 24h; | 99% |
| With potassium carbonate In acetone at 20 - 25℃; for 24h; | 99% |
| With potassium carbonate In acetone at 20℃; | 479 mg |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 70℃; for 16h; | 99% |
-
-
75560-39-3
1-(13)C-ethyl iodide

-
-
103-90-2
4-acetaminophenol

-
-
72156-72-0
N-4-((1-13C)Ethoxy)phenylacetamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 60h; | 98.6% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 12h; Reflux; | 98% |
| With potassium carbonate In butanone at 70℃; for 18h; Williamson Ether Synthesis; | 91% |
| With potassium carbonate In acetone for 48h; Heating; | 80% |
-
-
103-90-2
4-acetaminophenol

-
-
51288-37-0
N-(4-hydroxy-3-nitrophenyl)acetamide

| Conditions | Yield |
|---|---|
| With silica gel; citric acid; sodium nitrite In hexane at 20℃; for 2.25h; | 98% |
| With Nitrite In aq. acetate buffer at 25℃; pH=5; Electrochemical reaction; Green chemistry; regioselective reaction; | 85% |
| With sulfuric acid; guanidine nitrate In water at 0 - 5℃; Inert atmosphere; | 84% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In butanone for 24h; Reflux; | 98% |
| With potassium carbonate In butanone at 70℃; for 18h; Williamson Ether Synthesis; | 86% |
| With potassium carbonate In acetone for 18h; Heating; | 72% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; Inert atmosphere; | 98% |
| With triethylamine In dichloromethane at 0℃; for 2h; | 97% |
-
-
103-90-2
4-acetaminophenol

-
-
586-78-7
para-nitrophenyl bromide

-
-
2687-40-3
4-nitrophenyl 4'-acetamidophenyl ether

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 2-carbomethoxy-3-hydroxyquinoxaline-di-N-oxide; caesium carbonate In N,N-dimethyl-formamide at 110℃; for 11h; Schlenk technique; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 40℃; | 98% |
| With potassium carbonate In acetone for 4h; Reflux; | 97% |
| With potassium carbonate In acetone at 65℃; for 4h; Inert atmosphere; | 97% |
| With potassium carbonate In acetonitrile at 70℃; |
-
-
103-90-2
4-acetaminophenol

-
-
106-96-7
propargyl bromide

-
-
26557-77-7
N-(4-(prop-2-yn-1-yloxy)phenyl)acetamide

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile | 98% |
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 2h; | 95% |
| Stage #1: 4-acetaminophenol With sodium hydroxide In water at 70℃; Stage #2: propargyl bromide With tetrabutylammomium bromide In water; toluene at 70℃; for 24h; | 94% |
-
-
103-90-2
4-acetaminophenol

-
-
128175-80-4
2,2-dichloro-4,4,6,6-bis[spiro(2',2''-dioxy-1'',1''-biphenyl)]cyclotriphosphazene

-
-
165899-64-9
C40H32N5O8P3

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 48h; Reflux; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With ferric hydrogen sulphate; triethylamine at 20℃; for 60h; Inert atmosphere; chemoselective reaction; | 98% |
| With triethylamine at 20℃; Inert atmosphere; | 80% |
-
-
103-90-2
4-acetaminophenol

-
-
16704-37-3
4-acetamidophenyl sulfurofluoridate

| Conditions | Yield |
|---|---|
| With fluorosulfonyl fluoride; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 18h; | 98% |
| Stage #1: 4-acetaminophenol With triethylamine In acetonitrile at 20℃; for 0.166667h; Stage #2: With 1-(fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate In acetonitrile for 1h; | 98% |
| Stage #1: 4-acetaminophenol With triethylamine In acetonitrile at 20℃; for 0.166667h; Stage #2: With 1-(fluorosulfuryl)-2,3-dimethyl-1H-imidazol-3-ium trifluoromethanesulfonate In acetonitrile for 1h; Inert atmosphere; | 98% |
-
-
103-90-2
4-acetaminophenol

-
-
128175-79-1
1,1'-spiro-<2,2'-dioxybiphenyl-1,1'>-3,3',5,5'-tetrachloro-2,4,6,1λ5,3λ5,5λ5-triazatriphosphorine

-
-
1331732-22-9
C44H40N7O10P3

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 48h; Reflux; Inert atmosphere; | 97% |
Acetaminophen History
Harmon Northrop Morse had already synthesized paracetamol at Johns Hopkins University via the reduction of p-nitrophenol with tin in glacial acetic acid in 1877, but it wasn't until 1887 that clinical pharmacologist Joseph von Mering tried paracetamol on patients.
In 1893, von Mering published a paper reporting on the clinical results of paracetamol with phenacetin, another aniline derivative. Von Mering claimed that, unlike phenacetin, paracetamol had a slight tendency to produce methemoglobinemia.
Paracetamol was then quickly discarded in favor of phenacetin. The sales of phenacetin established Bayer as a leading pharmaceutical company.Overshadowed in part by aspirin, introduced into medicine by Heinrich Dreser in 1899, phenacetin was popular for many decades, particularly in widely advertised over-the-counter “headache mixtures,” usually containing phenacetin, an aminopyrine derivative or aspirin, caffeine, and sometimes a barbiturate.
In 1947 David Lester and Leon Greenberg found strong evidence that paracetamol was a major metabolite of acetanilide in human blood, and in a subsequent study they reported that large doses of paracetamol given to albino rats did not cause methemoglobinemia.
It has been suggested that contamination of paracetamol with 4-aminophenol, the substance from which it was synthesized by von Mering, may be the cause for his spurious findings.
In 1953, paracetamol was first marketed in the United States by Sterling-Winthrop Co.,.
In 1956, 500 mg tablets of paracetamol went on sale in the United Kingdom under the trade name Panadol, produced by Frederick Stearns & Co, a subsidiary of Sterling Drug Inc.
In 1963, paracetamol was added to the British Pharmacopoeia, and has gained popularity since then as an analgesic agent with few side-effects and little interaction with other pharmaceutical agents.
Concerns about paracetamol's safety delayed its widespread acceptance until the 1970s, but in the 1980s paracetamol sales exceeded those of aspirin in many countries, including the United Kingdom.
Acetaminophen Specification
The Acetaminophen with CAS registry number of 103-90-2 is also known as Acetamide,N-(4-hydroxyphenyl)-. The IUPAC name is N-(4-Hydroxyphenyl)acetamide. It belongs to product categories of Pharmaceuticals; Aromatic Phenols; Intermediates & Fine Chemicals; Lipid signaling. Its EINECS registry number is 203-157-5. In addition, the formula is C8H9NO2 and the molecular weight is 151.17. This chemical is a white crystalline powder that slightly soluble in water. It may cause damage to health and should be sealed in ventilated, cool room away from fire, heat. What's more, this chemical can be used as antipyretic, analgesic, anti-rheumatic drugs.
Physical properties about Acetaminophen are:
(1)ACD/LogP: 0.48; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.475; (4)ACD/LogD (pH 7.4): 0.474; (5)ACD/BCF (pH 5.5): 1.352; (6)ACD/BCF (pH 7.4): 1.348; (7)ACD/KOC (pH 5.5): 43.194; (8)ACD/KOC (pH 7.4): 43.061; (9)#H bond acceptors: 3; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Index of Refraction: 1.619; (13)Molar Refractivity: 42.406 cm3; (14)Molar Volume: 120.946 cm3; (15)Surface Tension: 52.801 dyne/cm; (16)Density: 1.25 g/cm3; (17)Flash Point: 188.354 °C; (18)Enthalpy of Vaporization: 66.186 kJ/mol; (19)Boiling Point: 387.831 °C at 760 mmHg; (20)Vapour Pressure: 0 mmHg at 25 °C.
Preparation of Acetaminophen:
Firstly, aminophenol and glacial acetic acid are added into dilute acetic acid by turns. Then the reaction mixture is heated to 150 °C for 7 hours. Secondly, adding acetic anhydride into mixture solution for another 2 hours. At last, product is obtained by rejection filtering, washing and drying. The yield is about 90%.
Uses of Acetaminophen:
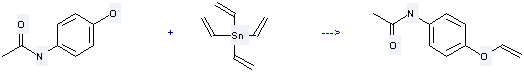
It is used to produce N-acetyl-p-vinyloxyaniline by reaction with tetravinylstannane. The reaction occurs with reagents Cu(OAc)2, O2 and solvent acetonitrile at 20 °C for 22 hours. The yield is about 90%.
Safety information of Acetaminophen:
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system, skin and is harmful if swallowed. This chemical is also harmful to aquatic organisms that may cause long-term adverse effects in the aquatic environment. However, there is limited evidence of a carcinogenic effect. During using it, wear suitable protective clothing, gloves and eye/face protection. Do not breathe dust and avoid release to the environment. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CC(=O)NC1=CC=C(C=C1)O
2. InChI: InChI=1S/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10)
3. InChIKey: RZVAJINKPMORJF-UHFFFAOYSA-N
The toxicity data of Acetaminophen is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | LDLo | oral | 50mg/kg (50mg/kg) | CARDIAC: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | American Journal of Emergency Medicine. Vol. 6, Pg. 510, 1988. |
| child | LDLo | oral | 140mg/kg/7D-I (140mg/kg) | BEHAVIORAL: COMA LIVER: "HEPATITIS (HEPATOCELLULAR NECROSIS), ZONAL" BLOOD: "CHANGES IN SERUM COMPOSITION (E.G., TP, BILIRUBIN, CHOLESTEROL)" | Medical Journal of Australia. Vol. 171, Pg. 472, 1999. |
| child | LDLo | oral | 360mg/kg/2D (360mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING LIVER: OTHER CHANGES SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Journal of Pediatrics. Vol. 92, Pg. 832, 1978. |
| child | TDLo | oral | 591mg/kg/2D-I (591mg/kg) | LIVER: LIVER FUNCTION TESTS IMPAIRED KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" BLOOD: APLASTIC ANEMIA | Clinical Pediatrics Vol. 33, Pg. 42, 1994. |
| child | TDLo | oral | 801mg/kg (801mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC GASTROINTESTINAL: NAUSEA OR VOMITING LIVER: OTHER CHANGES | Pediatrics. Vol. 61, Pg. 68, 1978. |
| dog | LDLo | intravenous | 826mg/kg (826mg/kg) | BEHAVIORAL: ANALGESIA | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 149, Pg. 571, 1964. |
| dog | LDLo | oral | 2gm/kg (2000mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BLOOD: CHANGES IN SPLEEN | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 24, Pg. 602, 1993. |
| frog | LDLo | subcutaneous | 50mg/kg (50mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BEHAVIORAL: ATAXIA | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 33, Pg. 216, 1894. |
| guinea pig | LD50 | oral | 2620mg/kg (2620mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: TREMOR BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Journal of the American Pharmaceutical Association, Scientific Edition. Vol. 47, Pg. 479, 1958. |
| human | LDLo | oral | 143mg/kg (143mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC | British Medical Journal. Vol. 282, Pg. 199, 1981. |
| human | LDLo | oral | 357mg/kg (357mg/kg) | BEHAVIORAL: ANOREXIA (HUMAN GASTROINTESTINAL: NAUSEA OR VOMITING BEHAVIORAL: COMA | Lancet. Vol. 1, Pg. 66, 1973. |
| infant | TDLo | oral | 1440mg/kg/6D (1440mg/kg) | BEHAVIORAL: IRRITABILITY GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | American Journal of Diseases of Children. Vol. 137, Pg. 386, 1983. |
| mammal (species unspecified) | LD50 | unreported | 891mg/kg (891mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 48(6), Pg. 22, 1983. | |
| mammal (species unspecified) | LDLo | oral | 512mg/kg (512mg/kg) | United States Patent Document. Vol. #4035499, | |
| man | LDLo | oral | 143mg/kg/24H- (143mg/kg) | BEHAVIORAL: ANOREXIA (HUMAN LIVER: "JAUNDICE, OTHER OR UNCLASSIFIED" LIVER: "HEPATITIS (HEPATOCELLULAR NECROSIS), ZONAL" | American Journal of Medicine. Vol. 74, Pg. 349, 1983. |
| man | LDLo | oral | 714mg/kg (714mg/kg) | LIVER: OTHER CHANGES | Human Toxicology. Vol. 1, Pg. 25, 1981. |
| man | TDLo | oral | 9286ug/kg (9.286mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE SENSE ORGANS AND SPECIAL SENSES: OTHER: EAR SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Allergy. Vol. 50(Suppl, |
| man | TDLo | oral | 714mg/kg (714mg/kg) | CARDIAC: EKG CHANGES NOT DIAGNOSTIC OF ABOVE | Postgraduate Medical Journal. Vol. 69, Pg. 52, 1993. |
| mouse | LD50 | intraperitoneal | 367mg/kg (367mg/kg) | BEHAVIORAL: ANALGESIA | Arzneimittel-Forschung. Drug Research. Vol. 15, Pg. 520, 1965. |
| mouse | LD50 | oral | 338mg/kg (338mg/kg) | Toxicology and Applied Pharmacology. Vol. 19, Pg. 20, 1971. | |
| mouse | LD50 | subcutaneous | 310mg/kg (310mg/kg) | Human Toxicology. Vol. 3, Pg. 13S, 1984. | |
| pig | LDLo | intravenous | 1gm/kg (1000mg/kg) | LIVER: OTHER CHANGES KIDNEY, URETER, AND BLADDER: OTHER CHANGES | Veterinary and Human Toxicology. Vol. 30, Pg. 324, 1988. |
| rat | LD50 | intraperitoneal | 1205mg/kg (1205mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: TREMOR | Studi Sassaresi, Sezione 2. Vol. 57, Pg. 561, 1979. |
| rat | LD50 | oral | 1944mg/kg (1944mg/kg) | United States Patent Document. Vol. #4636513, | |
| women | LDLo | oral | 260mg/kg (260mg/kg) | BEHAVIORAL: COMA GASTROINTESTINAL: NAUSEA OR VOMITING KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | JAMA, Journal of the American Medical Association. Vol. 236, Pg. 1874, 1976. |
| women | LDLo | oral | 400mg/kg (400mg/kg) | BEHAVIORAL: COMA LIVER: LIVER FUNCTION TESTS IMPAIRED | Journal of Toxicology, Clinical Toxicology. Vol. 35, Pg. 325, 1997. |
| women | LDLo | oral | 650mg/kg (650mg/kg) | VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION VASCULAR: OTHER CHANGES | American Journal of Emergency Medicine. Vol. 6, Pg. 511, 1988. |
| women | TDLo | oral | 4962ug/kg (4.962mg/kg) | GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF ENDOCRINE PANCREAS BLOOD: OTHER CHANGES LIVER: LIVER FUNCTION TESTS IMPAIRED | Journal of Toxicology, Clinical Toxicology. Vol. 29, Pg. 223, 1991. |
| women | TDLo | oral | 13mg/kg (13mg/kg) | BEHAVIORAL: EXCITEMENT | Allergy. Vol. 50(Suppl, |
| women | TDLo | oral | 325mg/kg (325mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | Postgraduate Medical Journal. Vol. 68, Pg. 116, 1992. |
| women | TDLo | oral | 490mg/kg (490mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: OTHER CHANGES KIDNEY, URETER, AND BLADDER: OTHER CHANGES | Southern Medical Journal. Vol. 71, Pg. 906, 1978. |
Related Products
- Acetaminophen
- Acetaminophen mercapturate
- 103904-10-5
- 1039046-54-2
- 1039055-46-3
- 103909-75-7
- 103914-52-9
- 103917-26-6
- 103918-71-4
- 103918-73-6
- 103-92-4
- 103933-26-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View