-
Name
Acetone semicarbazone
- EINECS 203-746-7
- CAS No. 110-20-3
- Article Data12
- CAS DataBase
- Density 1.171 g/cm3
- Solubility
- Melting Point 189-190 °C
- Formula C4H9N3O
- Boiling Point 215.41°C (rough estimate)
- Molecular Weight 115.135
- Flash Point
- Transport Information
- Appearance Clear liquid
- Safety 22-36/37
- Risk Codes 22
-
Molecular Structure
- Hazard Symbols R22:Harmful if swallowed.;
- Synonyms Acetone,semicarbazone (6CI,7CI,8CI);
- PSA 67.48000
- LogP 1.14170
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium acetate In water | 75% |
| With sodium acetate In water at 20℃; for 18h; | 75% |
| With sodium acetate In ethanol; water for 0.5h; | |
| With sodium acetate In water at 20℃; |

-
-
110-20-3
acetone semicarbazone

| Conditions | Yield |
|---|---|
| an der Luft; |

| Conditions | Yield |
|---|---|
| With acetic acid | |
| With alkali |
-
-
22123-17-7
4-isopropylidene-5-methyl-2-phenyl-2,4-dihydro-pyrazol-3-one

-
-
4426-72-6, 51433-48-8
hydrazine carboxamide

-
A
-
89-25-8
edaravone

-
B
-
110-20-3
acetone semicarbazone

| Conditions | Yield |
|---|---|
| With sodium acetate In ethanol for 1h; Product distribution; Ambient temperature; |

-
-
108161-18-8
5-Methyl-4-(1-methylethylidene)-2-(4'-chlorophenyl)-2,4-dihydro-3H-pyrazol-3-one

-
-
4426-72-6, 51433-48-8
hydrazine carboxamide

-
A
-
13024-90-3
1-(4-chlorophenyl)-3-methyl-2-pyrazolin-5-one

-
B
-
110-20-3
acetone semicarbazone

| Conditions | Yield |
|---|---|
| With sodium acetate In ethanol for 1h; Product distribution; Ambient temperature; |

-
-
85921-22-8
5-Methyl-4-(1-methylethylidene)-2-(4'-methoxyphenyl)-2,4-dihydro-3H-pyrazol-3-one

-
-
4426-72-6, 51433-48-8
hydrazine carboxamide

-
A
-
60798-06-3
1-(4-methoxyphenyl)-3-methyl-2-pyrazolin-5-one

-
B
-
110-20-3
acetone semicarbazone

| Conditions | Yield |
|---|---|
| With sodium acetate In ethanol for 1h; Product distribution; Ambient temperature; |

-
-
76973-30-3
5-Methyl-4-(1-methylethylidene)-2-(4'-nitrophenyl)-2,4-dihydro-3H-pyrazol-3-one

-
-
4426-72-6, 51433-48-8
hydrazine carboxamide

-
A
-
6402-09-1
1-(4'-nitrophenyl)-3-methyl-5-pyrazolone

-
B
-
110-20-3
acetone semicarbazone

| Conditions | Yield |
|---|---|
| With sodium acetate In ethanol for 1h; Product distribution; Ambient temperature; |

-
-
7732-18-5
water

-
-
563-41-7
semicarbazide hydrochloride

-
-
127-08-2
potassium acetate

-
-
627-70-3
propan-2-one azine

-
-
110-20-3
acetone semicarbazone

-
-
4426-72-6, 51433-48-8
hydrazine carboxamide

-
-
110-20-3
acetone semicarbazone

| Conditions | Yield |
|---|---|
| With water | |
| With ethanol | |
| With benzene |



-
-
96-34-4
methyl chloroacetate

-
-
110-20-3
acetone semicarbazone

-
-
61619-60-1
1-methyl-3-(5-nitro-furan-2-yl)-1H-pyrazole-4-carbaldehyde

-
-
61620-57-3
1-[1-methyl-3-(5-nitro-furan-2-yl)-1H-pyrazol-4-ylmethyleneamino]-imidazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| With hydrogenchloride In N-methyl-acetamide; water; isopropyl alcohol | 90% |


| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran byproducts: triethylamine hydrochloride; stirring for 25hours, filtration, evaporation; recryst., elem. anal.; | 90% |
-
-
141-43-5
ethanolamine

-
-
110-20-3
acetone semicarbazone

-
-
98278-63-8
acetone 4-(2-hydroxyethyl)semicarbazone

| Conditions | Yield |
|---|---|
| In toluene Heating; | 81% |
| In toluene for 7h; transamination; Heating; | 81% |
-
-
61676-63-9
2-isopropoxy-1,3,2-benzodioxaborole

-
-
110-20-3
acetone semicarbazone

-
-
90963-54-5
C6H4(O)2B(CH3)2CNNCONH2

| Conditions | Yield |
|---|---|
| In benzene byproducts: isopropanol; reflux for 8-10h, isopropanol was removed azeotropically, evaporation; TLC, elem. anal.; | 81% |
-
-
35238-97-2
Shrock-Osborn catalyst

-
-
75-09-2
dichloromethane

-
-
1333-74-0
hydrogen

-
-
67-64-1
acetone

-
-
110-20-3
acetone semicarbazone

| Conditions | Yield |
|---|---|
| In acetone byproducts: acetone; under Ar; Rh complex treated with H2 (1 atm) in acetone at room temp. for 2.5 h, semicarbazone added, stirred at room temp. for ca. 15 min; evapd. under vac. at room temp., residue dissolved in CH2Cl2, addn. of hexane or Et2O, ppt. collected, dried under vac. overnight; elem. anal.; | 78% |


| Conditions | Yield |
|---|---|
| In dichloromethane N2; room temp., equimolar quantities, 15 min;; filtered, washed with Et2O, dried in vacuo, elem. anal.;; | 76% |

| Conditions | Yield |
|---|---|
| In dichloromethane equimolar amts. of educts; pptn. (addn. of Et2O); elem. anal.; | 76% |
-
-
110-20-3
acetone semicarbazone

| Conditions | Yield |
|---|---|
| With lead(IV) acetate; potassium carbonate In dichloromethane for 0.5h; | 75% |
-
-
156-87-6
propan-1-ol-3-amine

-
-
110-20-3
acetone semicarbazone

-
-
176964-66-2
acetone 4-(3-hydroxypropyl)semicarbazone

| Conditions | Yield |
|---|---|
| In toluene Heating; | 60% |
| In toluene |

| Conditions | Yield |
|---|---|
| In toluene Heating; | 60% |
| In toluene Heating; |

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 4h; Cyclization; | 60% |

| Conditions | Yield |
|---|---|
| With HBr In ethanol; water hot EtOH/H2O soln. of salt mixed with EtOH soln. of ligand, excess of HBr added, mixt. refluxed until brown fumes ceased to come out; concd., cooled, filtered out, washed with 50% EtOH, dried at 60°C; elem. anal.; | 60% |

| Conditions | Yield |
|---|---|
| In toluene for 8.5h; Heating; | 59% |
-
-
126109-21-5, 125962-97-2, 135267-73-1
{WI(carbonyl)(acetonitrile)(dppm)(eta.2-but-2-yne)}{tetrafluoroborate}

-
-
110-20-3
acetone semicarbazone

| Conditions | Yield |
|---|---|
| In dichloromethane N2 atmosphere; addn. of org. compd. to soln. of W-compd. in CH2Cl2 with stirring (N2 stream),reacting (3 h); filtration, partial evapn. (vac.), pptn. on dropwise addn. of Et2O, recrystn. (CH2Cl2/Et2O); elem. anal.; | 59% |

| Conditions | Yield |
|---|---|
| Stage #1: [{Ir(μ-Cl)(2-(p-tolyl)pyridinato)2}2]; acetone semicarbazone With sodium methylate In methanol; dichloromethane at 20℃; for 3h; Stage #2: chloroform | 58% |

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
110-20-3
acetone semicarbazone

-
-
112758-40-4
5-methyl-1H-pyrazole-4-carbaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate at 0℃; for 0.25h; Stage #2: acetone semicarbazone at 70℃; for 4h; Stage #3: With hydrogenchloride; sodium hydroxide more than 3 stages; | 52.1% |
-
-
14778-29-1
1,1,2,2,-tetracyanoethane

-
-
110-20-3
acetone semicarbazone

-
-
140867-53-4
(5-Amino-3,3,4-tricyano-2,2-dimethyl-2,3-dihydro-pyrrol-1-yl)-urea

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide | 50% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 72h; Ugi reaction; | 50% |


-
-
110-20-3
acetone semicarbazone

-
-
86743-61-5
Co((CH3)2CNNHCONH2)2(NCS)2

| Conditions | Yield |
|---|---|
| In ethanol; water hot EtOH/H2O soln. of salt mixed with EtOH soln. of ligand, excess of ammonium thiocyanate soln. in H2O added, mixt. refluxed for 3 h; cooled, filtered out, washed with 50% EtOH, dried at 60°C; elem. anal.; | 50% |


| Conditions | Yield |
|---|---|
| In ethanol; water hot EtOH/H2O soln. of salt mixed with EtOH soln. of ligand, mixt. refluxed for ca. 1 h; cooled, filtered out, washed with 50% EtOH, dried at 60°C; elem. anal.; | 50% |

| Conditions | Yield |
|---|---|
| With H2SO4 In ethanol; water Co salt dissolved in min. H2O, few drops of concd. H2SO4 added, heated to 80°C, hot EtOH soln. of ligand added, soln. refluxed for ca. 3 h; concd., cooled, filtered, washed with 50% EtOH, dried at 60°C; elem. anal.; | 50% |

-
-
110-20-3
acetone semicarbazone

| Conditions | Yield |
|---|---|
| In ethanol hot ethanolic chromium salt was mixed with hot ethanolic ligand, mixt. was refluxed on a waterbath for about 4 h; mixt. was concd., cooled overnight, crystalls were filtered, washed with aq. EtOH, dried at ca. 60°C; elem. anal.; | 50% |
-
-
762-04-9
phosphonic acid diethyl ester

-
-
110-20-3
acetone semicarbazone

-
-
1415724-69-4
diethyl [1-(2-carbamoylhydrazino)-1-methylethyl]phosphonate

| Conditions | Yield |
|---|---|
| With tetra[tert-butylphthalocyanine]aluminum chloride at 80℃; for 20h; Inert atmosphere; | 50% |
-
-
931-53-3
Cyclohexyl isocyanide

-
-
7099-91-4
p-methoxybenzoylformic acid

-
-
110-20-3
acetone semicarbazone

-
-
898261-58-0
N-cyclohexyl-2-[N'-carbamoyl-N-(2-oxo-2-{p-methoxy}phenylacetyl)hydrazino]isobutyramide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 72h; Ugi reaction; | 48% |


-
-
110-20-3
acetone semicarbazone

-
-
84147-76-2
Cr(OC(NH2)NHNC(CH3)2)2Cl2(1+)*Cl(1-)=Cr(OC(NH2)NHNC(CH3)2)2Cl3

| Conditions | Yield |
|---|---|
| In ethanol hot ethanolic chromium salt was mixed with hot ethanolic ligand, mixt. was refluxed on a waterbath for about 4 h; mixt. was concd., cooled overnight, crystalls were filtered, washed with aq. EtOH, dried at ca. 60°C; elem. anal.; | 45% |
-
-
79-37-8
oxalyl dichloride

-
-
110-20-3
acetone semicarbazone

-
-
133559-55-4
1-Aminoimidazole-2,4,5-(1H,3H)-trione

| Conditions | Yield |
|---|---|
| In benzene for 4h; Cyclization; Heating; | 35% |
-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
110-20-3
acetone semicarbazone

-
-
112758-40-4
3-methyl-1H-pyrazole-4-carbaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate at 0℃; for 1.5h; Stage #2: acetone semicarbazone at 0 - 70℃; Stage #3: With sodium hydroxide In water Cooling with ice; | 32% |
| With sodium hydroxide; trichlorophosphate 1.) 60 deg C, 4 h, 2.) from 50 deg C to 60 deg C, 5 min; Yield given. Multistep reaction; |
-
-
110-20-3
acetone semicarbazone

-
-
30318-89-9
4-methyl-1,2,3-selenadiazole

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide; acetic acid for 20h; | 17% |
| With selenium(IV) oxide In acetic acid |
-
-
110-89-4
piperidine

-
-
108-88-3
toluene

-
-
110-20-3
acetone semicarbazone

-
-
876472-00-3
piperidine-1-carboxylic acid isopropylidenehydrazide

| Conditions | Yield |
|---|---|
| Erhitzen unter Ausschluss von Feuchtigkeit; |

Acetone semicarbazone Chemical Properties
Product Name: Acetone semicarbazone (CAS NO.110-20-3)
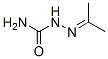
Molecular Formula: C4H9N3O
Molecular Weight: 115.13g/mol
H bond acceptors: 4
H bond donors: 3
Freely Rotating Bonds: 1
Polar Surface Area: 35.91Å2
Index of Refraction: 1.511
Molar Refractivity: 29.47 cm3
Molar Volume: 98.3 cm3
Surface Tension: 39.2 dyne/cm
Mol File: 110-20-3.mol
Einecs: 203-746-7
Melting Point: 189-190°C
Density: 1.17 g/cm3
Surface Tension: 39.2 dyne/cm
XLogP3-AA: 1.6
H-Bond Donor: 0
H-Bond Acceptor: 2
Product Categories: Pharmaceutical Intermediates
InChI
InChI=1/C4H9N3O/c1-3(2)6-7-4(5)8/h1-2H3,(H3,5,7,8)
Smiles
O=C(N\N=C(\C)C)N
Acetone semicarbazone Production
With hydrazine hydrate and urea as raw materials, condensation may semicarbazide solution, and then with acetone synthesis, refining was finished. Hydrazine hydrate and urea will be mixed, heated to 97-104 °C , return to 6-7h. Down to 45 °C , filtration, may semicarbazide solution. The filtrate cooling to 25 °C , by adding acetone, the natural temperature 50-60 °C , heat 1h. Cooling to 20 °C below, filtration, cake drying, may acetonide ammonia urea. Ingredients weight ratio: hydrazine hydrate (40%): Urea: acetone = 1:0.67:0.56. Yield of 90%. As intermediates, the appearance is white powder, melting point 185-189 °C . Consumption of raw materials fixed: hydrazine hydrate (40%) 300kg / t, urea (industrial) 870kg / t, acetone (industrial) 720kg / t.
Acetone semicarbazone Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 90mg/kg (90mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Journal of Pharmacology and Experimental Therapeutics. Vol. 122, Pg. 110, 1958. |
Acetone semicarbazone Consensus Reports
Reported in EPA TSCA Inventory.
Acetone semicarbazone Safety Profile
Poison by intravenous route. When heated to decomposition it emits toxic fumes of NOx.
Safety Information of Acetone semicarbazone (CAS NO.110-20-3):
Risk Statements: 22
22: Harmful if swallowed
Safety Statements: 22-36/37
22: Do not breathe dust
36: Wear suitable protective clothing
37: Wear suitable gloves
Acetone semicarbazone Specification
Acetone semicarbazone , its CAS NO. is 110-20-3, the synonyms is 2-(1-Methylethylidene)-hydrazinecarboxamid ; Hydrazinecarboxamide, 2-(1-methylethylidene)- ; Aurora ka-350 ; Acetone semicarbazone ; 2-Propanone semicarbazone ; Acetonesemicarbazone ; 2-Propanonesemicarbazone ; Acetone semicarbazon ; Acetone semecarbazone . Acetone semicarbazone (CAS NO.110-20-3) is used to produce pharmaceutical intermediates furan tannin.
Related Products
- Acetone
- Acetone cyanohydrin
- ACETONE DIETHYLSULFONE
- Acetone diphenylamine
- Acetone oxime
- Acetone semicarbazone
- Acetone sodium bisulfite
- Acetone thiosemicarbazone
- 11021-13-9
- 11021-14-0
- 1102-19-8
- 110220-87-6
- 110221-26-6
- 110221-44-8
- 110223-15-9
- 110230-36-9
- 110230-98-3
- 110231-30-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View