 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
-
Name
Acetone
- EINECS 200-662-2
- CAS No. 67-64-1
- Article Data2732
- CAS DataBase
- Density 0.773 g/cm3
- Solubility soluble in water
- Melting Point -95--93 °C, 178-180 K, -139--136 °F
- Formula C3H6O
- Boiling Point 56-57 °C, 329-330 K, 133-134 °F
- Molecular Weight 58.08
- Flash Point -17 °C
- Transport Information UN 1090 3/PG 2
- Appearance clear colorless liquid
- Safety 9-16-26
- Risk Codes 11-36-66-67
-
Molecular Structure
-
Hazard Symbols
 F,
F, Xi
Xi
- Synonyms Dimethyl ketone;Dimethylformaldehyde;NSC 135802;Propanone;Pyroacetic ether;beta-Ketopropane;Acetone(8CI);Methyl ketone (6CI);2-Propanone;
- PSA 17.07000
- LogP 0.59530
Synthetic route

| Conditions | Yield |
|---|---|
| With trans-4L1(O)2>ClO4 In acetonitrile at 25℃; for 7h; stoicheiometric oxidation ( electrochemical oxidation in a non-aqueous medium (acetonitrile), an Ag-AgNO3 reference electrode; | 100% |
| With C19H20N3O2Ru(2+)*2F6P(1-) In aq. buffer at 24.84℃; for 1h; pH=1.8; Thermodynamic data; Activation energy; Reagent/catalyst; | 100% |
| With tert-butylethylene; C32H52ClIrP2; sodium t-butanolate at 200℃; for 2h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Glovebox; | 100% |
-
-
110-86-1
pyridine

-
-
31562-43-3
tert-butylsulfinyl chloride

-
A
-
628-13-7
pyridine hydrochloride

-
B
-
67-64-1
acetone

-
D
-
75-65-0
tert-butyl alcohol

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In chloroform at 20℃; for 2h; Further byproducts given; | A 100% B 20% C 40% D 55% |


| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; water; potassium carbonate; triphenylbismuthane In acetonitrile for 2h; | 100% |
| With tert.-butylhydroperoxide; bis(acetylacetonato)dioxidomolybdenum(VI) In chlorobenzene at 60℃; for 24h; | 99% |
| With tert.-butylhydroperoxide; chromium tetra(tert-butoxide) In benzene at 20℃; for 24h; | 90% |
-
-
3968-30-7
5-cyclopentylidene-2,2-dimethyl-1,3-dioxane-4,6-dione

-
A
-
2873-50-9
butatriene

-
B
-
74-85-1
ethene

-
C
-
124-38-9
carbon dioxide

-
D
-
1165952-91-9
cyclohexa-1,3-diene

-
E
-
67-64-1
acetone

-
F
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| With variation of temp. at 550℃; Product distribution; | A 4% B 11.9% C 100% D 39.2% E 101.9 % F 3.3% |

-
-
110-82-7
cyclohexane

-
-
84210-61-7
perpentene-4 oate de tertiobutyle

-
A
-
92-51-3
cyclohexylcyclohexane

-
B
-
1678-93-9
butylcyclohexane

-
C
-
96009-79-9
cyclohexyl-5 pentanolide-4

-
D
-
67-64-1
acetone

-
E
-
108-29-2
5-methyl-dihydro-furan-2-one

-
F
-
75-65-0
tert-butyl alcohol

| Conditions | Yield |
|---|---|
| at 120℃; for 4h; Product distribution; Mechanism; different ratios of reactant, reactants, reaction times and temperatures; | A 5% B 5% C 35% D n/a E 1% F 100% |

-
-
110-82-7
cyclohexane

-
-
80037-90-7
1,1,3,3-tetramethyl-2,3-dihydro-1H-isoindol-2-yloxoyl radical

-
-
37421-16-2
2-(t-butylazo)prop-2-yl hydroperoxide

-
A
-
89482-40-6
2-cyclohexyloxy-1,1,3,3-tetramethyl-2,3-dihydro-1H-isoindole

-
B
-
93524-81-3
2-tert-butoxy-1,1,3,3-tetramethylisoindoline

-
C
-
67-64-1
acetone

-
D
-
115-11-7
isobutene

| Conditions | Yield |
|---|---|
| at 70℃; for 17h; Mechanism; Rate constant; Thermodynamic data; var. of nitroxide, solvent, temp., EA, ΔH(excit.), ΔS(excit.); | A 96% B 82% C 100% D 15% |

-
-
83073-73-8
2-(octylsulfonyl)ethyl tert-butyl peroxide

-
A
-
34557-54-5
methane

-
B
-
20466-47-1
2-hydroxyethyl octyl sulfone

-
C
-
67-64-1
acetone

-
D
-
75-65-0
tert-butyl alcohol

| Conditions | Yield |
|---|---|
| In various solvent(s) at 145℃; Rate constant; Thermodynamic data; E(act.); | A n/a B 100% C n/a D n/a |

-
-
120711-57-1
H-Dmt-Gly-Gly-Ala-OH trifluoroacetate salt

-
A
-
120711-58-2
H-Cys-Gly-Gly-Ala-OH

-
B
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| In ethanol; water Heating; | A 100% B n/a |

| Conditions | Yield |
|---|---|
| With potassium carbonate; Aliquat 336 In neat (no solvent) at 70℃; for 0.5h; | A n/a B 100% |
| With potassium carbonate; Aliquat 336 In neat (no solvent) at 70℃; for 0.5h; Product distribution; | A n/a B 100% |

-
-
34107-52-3
2,2-dimethyl-5-(phenyl-λ3-iodaneylidene)-1,3-dioxane-4,6-dione

-
A
-
2033-24-1
cycl-isopropylidene malonate

-
B
-
591-50-4
iodobenzene

-
C
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate In dichloromethane at 20℃; Product distribution; | A 33% B 100% C 5% |


-
A
-
67552-35-6, 79390-59-3, 79042-98-1
NiCH3(N(CO)2C6H4)(P(C2H5)3)2

-
B
-
14917-14-7
Ni(CO)2(2,2'-bipyridine)

-
C
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| In benzene-d6 40°C, 72 h; | A 100% B n/a C 33% |


| Conditions | Yield |
|---|---|
| In 1,4-dioxane; benzene-d6 Kinetics; 100°C (10.25 h); | A 100% B 97% |
| In benzene-d6 Irradiation (UV/VIS); | A 55% B n/a |


| Conditions | Yield |
|---|---|
| With CO In dichloromethane-d2 Irradiation (UV/VIS); 355-385 nm; 20 atm CO;; | A 100% B n/a C n/a D n/a |


| Conditions | Yield |
|---|---|
| With CO In benzene-d6 Irradiation (UV/VIS); 355-385 nm;; | A 100% B 68% C 34% D 10% |


| Conditions | Yield |
|---|---|
| With CO In dichloromethane-d2 Irradiation (UV/VIS); 20 atm CO;; | A 100% B 79% C 5% |


| Conditions | Yield |
|---|---|
| With carbon monoxide In dichloromethane-d2 Irradiation (UV/VIS); Irradiation of complex at 350-380 nm under 20 atm of CO in CD2Cl2 at 6-8°C;; | A 100% B 80% C 8% |
| With CO In 1,4-dioxane; dichloromethane-d2 Irradiation (UV/VIS); ca. 20 atm CO; 355-385 nm light, 22 min.; | A 100% B 78% C 6% |


| Conditions | Yield |
|---|---|
| With air In [D3]acetonitrile at 22℃; for 1h; UV-irradiation; Inert atmosphere; | A 100% B 41.9% C 25.9% |


| Conditions | Yield |
|---|---|
| at 250℃; for 0.666667h; Heating; Inert atmosphere; | 99.76% |

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In benzene for 0.916667h; Product distribution; Mechanism; Irradiation; varying reaction time; | A 88% B 99% |

| Conditions | Yield |
|---|---|
| With cis-[Ru(PPh2)(CH2)5PPh2(ampi)Cl2]; potassium hydroxide at 80℃; for 0.5h; Reagent/catalyst; Concentration; Schlenk technique; | A 99% B n/a |
| Stage #1: isopropyl alcohol With dichloro(1,5-cyclooctadiene)ruthenium(II); (R)-(6-methoxyquinolin-4-yl)((1S,2R,4S,5R)-5-vinylquinuclidin-2-yl)methanamine at 20℃; for 0.5h; Inert atmosphere; Stage #2: With potassium hydroxide for 0.5h; Inert atmosphere; Stage #3: acetophenone at 0℃; for 96h; Inert atmosphere; enantioselective reaction; | A 83% B n/a |
| With potassium hydroxide; oxalyl (1R,2R)-N,N'-bis[2-(amino)cyclohexyl]diamide; tris(triphenylphosphine)ruthenium(II) chloride at 20℃; for 24h; Product distribution; Further Variations:; Reagents; |


| Conditions | Yield |
|---|---|
| With [RuCl2(triphenylphosphine)((6-((3,5-dimethyl-pyrazol-1-yl)pyridin-2-yl)methylene)-p-tolyl-amine)]*0.5Et2O; potassium isopropoxide at 82℃; under 750.075 Torr; for 5.16667h; Inert atmosphere; | A 99% B n/a |
| With sodium hydroxide; RuCl2(PPh3)(iBu-BTP) at 82℃; under 750.075 Torr; for 9h; | A 97 % Chromat. B n/a |


| Conditions | Yield |
|---|---|
| With Fe(3+) In water; isopropyl alcohol Kinetics; byproducts: H(1+); excess of Fe(3+) in 1 M aq. i-PrOH at 24.8°C under N2 by controlled ionic strength; | A n/a B 99% |

-
B
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With gold(III) chloride In 1,2-dichloro-ethane at 25℃; Reagent/catalyst; Solvent; Sealed tube; | A 99% B n/a |
| With N-iodo-succinimide In 1,2-dichloro-ethane at 20℃; for 24h; Reagent/catalyst; | A 66% B n/a |

-
B
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With gold(III) chloride In 1,2-dichloro-ethane at 25℃; Sealed tube; | A 99% B n/a |

| Conditions | Yield |
|---|---|
| With iron(III) perchlorate In acetonitrile Product distribution; var. act.: CuClO4; var. solv.: CH2Cl2, toluene; var. conc.; determination of half life; | A 98.9% B 96.3% |
| With N-Phenyl-2-naphthylamine; cobalt(II) phthalocyanine In decalin at 40℃; Rate constant; Kinetics; var. temperatures, var. catalysts, var. solvents, without NA; | |
| In chlorobenzene at 75℃; Kinetics; various phosphites; |

| Conditions | Yield |
|---|---|
| 6xSAlH (sulfated alumina) at 246.85℃; Product distribution; Further Variations:; Catalysts; atmospheric pressure; | A 98.4% B 1.6% |
| bismuth molybdate; bismuth molybdate at 190℃; Product distribution; with Bi2Mo2O9 (β-phase) catalyst; | A 12% B 88% |
| With cobalt ferrite; oxygen at 299.84℃; for 3h; Autoclave; | A 52% B 40% |
-
-
75-91-2
tert.-butylhydroperoxide

-
-
30931-67-0
2,2'-azinobis-(3-ethyl-2,3-dihydrobenzothiazole-6-sulphonate) diammonium salt

-
A
-
67-56-1
methanol

-
B
-
50-00-0
formaldehyd

-
D
-
67-64-1
acetone

-
E
-
75-65-0
tert-butyl alcohol

| Conditions | Yield |
|---|---|
| Fe(III)T4MPyP In water at 30℃; Rate constant; Kinetics; Mechanism; the catalyst Fe(III)T4MPyP is 5,10,15,20-tetra(N-methyl-4-pyridyl)-porphyrinatoiron(III) pentachloride; pH 9.2; investigation of the dependence of velocity constant on ionic strength, pH and t-butyl hydroperoxide concentration; | A 3% B n/a C 72% D 4% E 98% |

-
-
87051-12-5
(4aRS,7aRS)-4a,7a-dihydro-3,3-dimethyl-6,7a-diphenyl-7H-cyclopenta<1,2-e><1,2,4>trioxine

-
-
114390-60-2
(1RS,2RS)-1,4-diphenylcyclopent-3-en-1,2 diol

-
B
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With acetic acid; zinc at 16℃; for 0.333333h; | A 98% B n/a |
-
-
99-91-2
para-chloroacetophenone

-
-
67-63-0
isopropyl alcohol

-
A
-
3391-10-4
1-(p-chlorophenyl)ethyl alcohol

-
B
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With C58H49ClN5P2Ru(1+)*Cl(1-); potassium isopropoxide at 82℃; for 0.00555556h; Inert atmosphere; | A 98% B n/a |
| With sodium hydroxide; RuCl2(PPh3)(iBu-BTP) at 82℃; under 750.075 Torr; for 30h; | A 89 % Chromat. B n/a |
| With [RuCl2(triphenylphosphine)((6-((3,5-dimethyl-pyrazol-1-yl)pyridin-2-yl)methylene)-p-tolyl-amine)]*0.5Et2O; potassium isopropoxide at 82℃; under 750.075 Torr; for 1.16667h; Inert atmosphere; | A 98 %Chromat. B n/a |


| Conditions | Yield |
|---|---|
| With carbon monoxide In diethyl ether Et2O soln. of Ni complex stirred under CO at -78°C for 0.2 h, warmed to room temp.; drying up; GLC anal.; | A n/a B 0% C 0% D 98% |


| Conditions | Yield |
|---|---|
| With FeOx-pillared bentonite at 30℃; for 0.0833333h; Reagent/catalyst; Time; Temperature; Solvent; | 100% |
| erbium(III) triflate at 20℃; for 0.5h; | 99% |
| With trichloro(trifluoromethanesulfonato)titanium(IV); n-tetrabutylammonium hydroxide In water for 0.166667h; Ambient temperature; | 98% |
-
-
120-57-0
piperonal

-
-
67-64-1
acetone

-
-
108439-88-9
(1E,4E)-1,5-bis(benzo[d][1,3]dioxol-5-yl)penta-1,4-dien-3-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 0.00833333h; Irradiation; | 100% |
| With sodium hydroxide In ethanol; water at 20℃; | 85% |
| With sodium hydroxide In ethanol; water at 20℃; Aldol Addition; | 79% |
-
-
555-16-8
4-nitrobenzaldehdye

-
-
67-64-1
acetone

-
-
88958-64-9, 88958-65-0, 97600-21-0, 57548-40-0
1-(4-nitrophenyl)-1-hydroxy-3-butanone

| Conditions | Yield |
|---|---|
| With Zn(2+)-(TyrOEt)2 In water at 40℃; for 24h; pH 7; | 100% |
| With sodium hydroxide at 25℃; for 0.166667h; | 100% |
| With Fe(OH)3/Fe3O4 at 50℃; for 3h; | 99% |
-
-
2004-06-0
6-Chloropurine riboside

-
-
67-64-1
acetone

-
-
39824-26-5
6-chloro-9-(2,3-O-isopropylidene-β-D-ribofuranosyl)-9H-purine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 20℃; for 3h; | 100% |
| In perchloric acid for 3h; Ambient temperature; | 94% |
| With perchloric acid at 25℃; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: cyclopenta-1,3-diene; acetone With pyrrolidine In methanol at 20℃; Inert atmosphere; Stage #2: With acetic acid In methanol for 0.15h; | 100% |
| With pyrrolidine In methanol at -10 - 20℃; for 1h; Inert atmosphere; | 100% |
| With methylamine for 4.5h; | 92% |
-
-
123-08-0
4-hydroxy-benzaldehyde

-
-
67-64-1
acetone

-
-
3654-49-7
(1E,4E)-1,5-bis(4-hydroxyphenyl)penta-1,4-dien-3-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran for 264h; | 100% |
| With hydrogenchloride; acetic acid at 25 - 30℃; for 2h; Heating; | 95% |
| With hydrogenchloride In acetic acid at 25 - 30℃; for 2h; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 120℃; under 6750.68 Torr; Aldol condensation; Continuous flow; | 100% |
| With 1-n-butyl-3-methylimidazolim bromide; bovine serum albumin at 60℃; for 6h; Aldol Condensation; Green chemistry; Enzymatic reaction; | 98% |
| With sodium hydroxide for 72h; Ambient temperature; | 97% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 20℃; | 100% |
| Stage #1: acetone With sodium hydroxide In water at 20℃; Microfluidic conditions; Stage #2: pivalaldehyde; acetone In water at 20℃; Microfluidic conditions; | 100% |
| With n-butyllithium; diisopropylamine In tetrahydrofuran at -78℃; for 0.583333h; | 47% |

| Conditions | Yield |
|---|---|
| In cyclohexane at 55℃; for 16h; | 100% |
| In hexane Reflux; | 93% |
| In hexane for 4h; Heating; | 91% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water at 20℃; for 0.5h; | 100% |
| With boehmite at 55℃; for 24h; Aldol Condensation; Green chemistry; | 55% |
| With sodium hydroxide |
-
-
93-02-7
2,5-dimethoxybenzaldehyde

-
-
67-64-1
acetone

-
-
118709-30-1
trans-1-(2,5-dimethoxyphenyl)-2-buten-3-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 23℃; for 6.5h; | 100% |
| Stage #1: 2,5-dimethoxybenzaldehyde; acetone With L-proline In dimethyl sulfoxide at 20℃; for 48h; Stage #2: With hydrogenchloride In water; dimethyl sulfoxide for 3h; | 62.5% |
| With sodium hydroxide at 20℃; for 0.5h; | 53% |

| Conditions | Yield |
|---|---|
| phosphazene base-P4-tert-butyl In hexane; dimethyl sulfoxide at 120℃; for 24h; | 100% |
| Stage #1: phenylacetylene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Inert atmosphere; Stage #2: acetone In tetrahydrofuran; hexane at -78 - 20℃; | 100% |
| With Nd(3+)*8Na(1+)*10C4H9O(1-)*HO(1-) In dimethyl sulfoxide at 30℃; for 24h; Catalytic behavior; Concentration; Solvent; Reagent/catalyst; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With cis-(Cl,Cl)-[Re(p-NC6H4CH3)Cl2(py-2-COO)(PPh3)] at 70℃; for 24h; Inert atmosphere; | 100% |
| With microporous zeolite at 230℃; for 24h; Sealed tube; Autoclave; | 97% |
| sodium hydrogen sulfate; silica gel at 50 - 52℃; for 0.0333333h; microwave irradiation; | 75% |

| Conditions | Yield |
|---|---|
| at 0℃; for 2h; gas/solid reaction; | 100% |
| Stage #1: 2-amino-benzenethiol; acetone In neat (no solvent) at 50℃; for 1h; Green chemistry; Stage #2: With o-benzenedisulfonimide In neat (no solvent) at 50℃; for 48h; Green chemistry; | 87% |
| With aluminum oxide at 20℃; for 0.5h; | 84% |
-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
67-64-1
acetone

-
-
5432-53-1
4-p-dimethylaminophenyl-3-buten-2-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 72h; Ambient temperature; | 100% |
| With water; potassium hydroxide at 20℃; for 0.2h; | 82% |
| With sodium hydroxide In water at 0 - 20℃; Aldol condensation; | 67.73% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane at 25℃; | 100% |
| With titanium tetrachloride | 79% |
| acid | 78% |

| Conditions | Yield |
|---|---|
| With cyclohexane at 105℃; for 1h; Dean-Stark; | 100% |
| With Amberlyst 36 at 50℃; for 2h; | 75% |
| With 4 A molecular sieve; Amberlyst A 15 In tetrahydrofuran for 24h; Ambient temperature; | 35% |
-
-
5973-71-7
3,4-dimethylbenzaldehyde

-
-
67-64-1
acetone

-
-
97241-86-6
(E)-4-(3,4-dimethylphenyl)but-3-en-2-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 0 - 25℃; for 6h; | 100% |
| With sodium hydroxide | |
| With sodium hydroxide In water at 25℃; for 12h; |
-
-
5906-99-0
2-nitrobenzenesulfonyl hydrazide

-
-
67-64-1
acetone

-
-
6655-27-2
N-isopropylidene-N’-2-nitrobenzenesulfonyl hydrazine

| Conditions | Yield |
|---|---|
| at 0℃; for 1h; | 100% |
| at 24℃; for 0.166667h; Inert atmosphere; | 95% |
| at 0 - 23℃; | 89% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 40℃; Reagent/catalyst; | 100% |
| With toluene-4-sulfonic acid In hexane at 70℃; for 12h; Dean-Stark; Molecular sieve; Inert atmosphere; | 100% |
| Acidic conditions; | 100% |
-
-
67-64-1
acetone

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
30983-80-3
(1E,4E)-1,5-bis(3,4-dichlorophenyl)penta-1,4-dien-3-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 20℃; for 1h; | 100% |
| With sodium hydroxide In ethanol; water at 20℃; Aldol Addition; | 70% |
| With sodium hydroxide | |
| With sodium hydroxide In ethanol |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; for 1h; | 100% |
| Stage #1: acetone; uridine With sulfuric acid at 20℃; for 1h; Stage #2: With triethylamine In acetone Product distribution / selectivity; | 100% |
| With sulfuric acid at 20℃; for 1h; | 100% |
-
-
67-64-1
acetone

-
-
4114-31-2
ethylhydrazine carboxylate

-
-
6637-60-1
N'-isopropylidenehydrazinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| 100% | |
| With magnesium sulfate Reflux; | 100% |
| for 2h; Heating; | 98% |
-
-
67-64-1
acetone

-
-
19214-95-0
(2-2H)propan-2-(2H)ol

| Conditions | Yield |
|---|---|
| With lithium aluminium deuteride In diethylene glycol dimethyl ether at 0℃; for 1h; Inert atmosphere; | 100% |
| With pyrographite; platinum; deuterium at 25℃; under 1520 Torr; an mit Eisensalz; | |
| With lithium aluminium deuteride |

| Conditions | Yield |
|---|---|
| With dinitrogen tetraoxide In tetrachloromethane 1.) 0 deg C, 20 min, 2.) 20 deg C, 40 min; | 100% |
| With nitric acid |

| Conditions | Yield |
|---|---|
| With hydrogen; mer-Os(PPh3)3HBr(CO) In toluene at 150℃; under 51680 Torr; for 3h; | 100% |
| With hydrogen; sodium methylate; chromium(0) hexacarbonyl In methanol at 120℃; under 75006 Torr; for 3h; | 100% |
| With hydrogen; Ru((R,R)-cyP2N2)HCl In benzene-d6 at 20℃; under 2280.15 Torr; for 12h; Product distribution / selectivity; Alkaline conditions; Cooling with liquid nitrogen; | 100% |
-
-
67-64-1
acetone

-
-
18449-76-8
methyl α-D-lyxopyranoside

-
-
60562-98-3
methyl 2,3-O-isopropylidene-α-D-lyxopyranoside

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; amberlyst-15 (H form) at 23℃; for 4h; | 100% |
| With sulfuric acid; copper(II) sulfate for 24h; Ambient temperature; | 81% |
| With sulfuric acid | |
| With phosphorus pentoxide |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; orthoformic acid triethyl ester at 20℃; | 100% |
| With p-toluenesulfonic acid monohydrate for 3h; Inert atmosphere; | 100% |
| With toluene-4-sulfonic acid at 20℃; for 1h; Inert atmosphere; | 99% |
-
-
100-52-7
benzaldehyde

-
-
67-64-1
acetone

-
-
5381-93-1, 86734-67-0, 86734-69-2, 127707-68-0
1-hydroxy-1-phenyl-3-butanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 20℃; | 100% |
| Stage #1: acetone With sodium hydroxide In water at 20℃; Microfluidic conditions; Stage #2: benzaldehyde; acetone In water at 20℃; Microfluidic conditions; | 100% |
| With pyrrolidine; 4-nitro-phenol at 20℃; for 2.5h; | 99.4% |
Acetone Specification
Acetone, with the CAS register number 67-64-1, is a clear colorless liquid with a sweetish odor. The substance which is the organic compound with the formula C3H6O has EINECS register number 200-662-2. Acetone is soluble in water. Acetone can form explosive mixtures with chromic anhydride, chromyl alcohol, hexacholromelamine, hydrogen peroxide, permonosulfuric acid, potassium terbutoxide and thioglycol. You should keep Acetone away from heat, sparks and flame. What's more, Acetone should be stored in a cool, dry place, and its container should be kept closed when not in use. In addition, Acetone can be restored by reductant into isopropyl alcohol and pinacolone. Acetone is relatively stable with oxidan, however, reacts with strong oxidizer oxidation, like alkaline potassium permanganate or chromium acid, can generate acetic acid, formic acid, carbon dioxide and water.
Physical properties about Acetone are: (1)ACD/LogP: -0.042; (2)ACD/LogD (pH 5.5): -0.04; (3)ACD/LogD (pH 7.4): -0.04; (4)ACD/BCF (pH 5.5): 1.00; (5)ACD/BCF (pH 7.4): 1.00; (6)ACD/KOC (pH 5.5): 22.61; (7)ACD/KOC (pH 7.4): 22.61; (8)#H bond acceptors: 1; (9)Index of Refraction: 1.345; (10)Molar Refractivity: 15.977 cm3; (11)Molar Volume: 75.176 cm3; (12)Polarizability: 6.334 10-24cm3; (13)Surface Tension: 18.818000793457 dyne/cm; (14)Density: 0.773 g/cm3; (15)Flash Point: -17.222 °C; (16)Enthalpy of Vaporization: 29.1 kJ/mol; (17)Boiling Point: 46.458 °C at 760 mmHg; (18)Vapour Pressure: 348.445007324219 mmHg at 25°C
Preparation of Acetone: Previously, Acetone was produced by the dry distillation of acetates, for example calcium acetate. During World War I, Acetone was produced via bacterial fermentation, as developed by Chaim Weizmann in order to help the British war effort. This Acetone Butanol Ethanol process was abandoned due to the small yields. Nowadays, Acetone is produced directly or indirectly from propylene. Approximately 83 % of Acetone is produced via the cumene process, as a result, acetone production is tied to phenol production. In the cumene process, benzene is alkylated with propylene and the resulting cumene (isopropylbenzene) is oxidized by air to give phenol and Acetone:
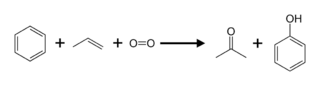
Besides, Acetone can be produced by acetylene with water vapor using zinc oxide as the catalyst.
2 C2H2 + 3 H2O → (CH3)2CO + CO2↑ + 2 H2↑
In addition, oxidation or dehydrogenation of isopropyl alcohol can be used to prepare Acetone. The isopropyl alcohol reacts with hydrogen peroxide to give Acetone. The isopropyl alcohol and acraldehyde can be used to generate Acetone.
Uses of Acetone: Acetone is also used extensively as a solvent for the safe transporting and storing of acetylene, which cannot be safely pressurized as a pure compound. Acetone is used in a variety of general medical and cosmetic applications and is also listed as a component in food additives and food packaging. In the laboratory, Acetone is used as a polar aprotic solvent in a variety of organic reactions, such as SN2 reactions.It also can be used as the active ingredient in nail polish remover and as paint thinner. Acetone is a good solvent for most plastics and synthetic fibers including those used in laboratory bottles made of polystyrene, polycarbonate and some types of polypropylene. It also can be used as a solvent and production of methyl methacrylate and bisphenol A. The procedures of producing methyl methacrylate and bisphenol A are as the following:
(CH3)2CO + HCN → (CH3)2C(OH)CN
(CH3)2C(OH)CN + CH3OH → CH2=(CH3)CCO2CH3 + NH3
(CH3)2CO + 2 C6H5OH → (CH3)2C(C6H4OH)2 + H2O
When you are using Acetone, you should be very cautious about it. Acetone is highly flammable and irritant, which is irritating to eyes. If repeated exposure, Acetone may cause skin dryness or cracking. Its vapours may cause drowsiness and dizziness. In addition, you should keep its container in a well-ventilated place and keep Acetone away from sources of ignition - No smoking.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC(=O)C
(2)InChI: InChI=1S/C3H6O/c1-3(2)4/h1-2H3
(3)InChIKey: CSCPPACGZOOCGX-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | intraperitoneal | 8gm/kg (8000mg/kg) | BEHAVIORAL: COMA GASTROINTESTINAL: ALTERATION IN GASTRIC SECRETION | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 18, Pg. 218, 1884. |
| dog | LDLo | oral | 8gm/kg (8000mg/kg) | BEHAVIORAL: ATAXIA BEHAVIORAL: COMA MUSCULOSKELETAL: JOINTS | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 18, Pg. 218, 1884. |
| dog | LDLo | subcutaneous | 5gm/kg (5000mg/kg) | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 18, Pg. 218, 1884. | |
| guinea pig | LD50 | skin | > 9400uL/kg (9.4mL/kg) | Toxicology and Applied Pharmacology. Vol. 7, Pg. 559, 1965. | |
| guinea pig | LDLo | subcutaneous | 5gm/kg (5000mg/kg) | Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 5, Pg. 1, 1933. | |
| human | TCLo | inhalation | 500ppm (500ppm) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 282, 1943. |
| man | LDLo | unreported | 1159mg/kg (1159mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| man | TCLo | inhalation | 440ug/m3/6M (0.44mg/m3) | BRAIN AND COVERINGS: RECORDINGS FROM SPECIFIC AREAS OF CNS | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 42(8), Pg. 42, 1977. |
| man | TCLo | inhalation | 10mg/m3/6H (10mg/m3) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 42(8), Pg. 42, 1977. | |
| man | TCLo | inhalation | 12000ppm/4H (12000ppm) | GASTROINTESTINAL: NAUSEA OR VOMITING BEHAVIORAL: MUSCLE WEAKNESS | Annals of Occupational Hygiene. Vol. 16, Pg. 73, 1973. |
| man | TDLo | oral | 2857mg/kg (2857mg/kg) | BEHAVIORAL: COMA KIDNEY, URETER, AND BLADDER: OTHER CHANGES | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 64, 1969. |
| man | TDLo | oral | 2857mg/kg (2857mg/kg) | BEHAVIORAL: COMA | Diabetes. Vol. 15, Pg. 810, 1966. |
| mouse | LC50 | inhalation | 44gm/m3/4H (44000mg/m3) | Current Toxicology. Vol. 1, Pg. 47, 1993. | |
| mouse | LD50 | intraperitoneal | 1297mg/kg (1297mg/kg) | Shell Chemical Company. Unpublished Report. Vol. -, Pg. 1, 1961. | |
| mouse | LD50 | oral | 3gm/kg (3000mg/kg) | Pharmaceutical Chemistry Journal Vol. 14, Pg. 162, 1980. | |
| mouse | LDLo | intravenous | 4gm/kg (4000mg/kg) | FAO Nutrition Meetings Report Series. Vol. 48A, Pg. 86, 1970. | |
| rabbit | LD50 | oral | 5340mg/kg (5340mg/kg) | FAO Nutrition Meetings Report Series. Vol. 48A, Pg. 86, 1970. | |
| rabbit | LDLo | intravenous | 1576mg/kg (1576mg/kg) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Journal of Pharmacology and Experimental Therapeutics. Vol. 33, Pg. 175, 1928. |
| rabbit | LDLo | skin | 20mL/kg (20mL/kg) | Union Carbide Data Sheet. Vol. 5/7/1970, | |
| rat | LC50 | inhalation | 50100mg/m3/8H (50100mg/m3) | American Industrial Hygiene Association Journal. Vol. 20, Pg. 364, 1959. | |
| rat | LD50 | intravenous | 5500mg/kg (5500mg/kg) | Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 1, 1974. | |
| rat | LD50 | oral | 5800mg/kg (5800mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: TREMOR | Journal of Toxicology and Environmental Health. Vol. 15, Pg. 609, 1985. |
| rat | LDLo | intraperitoneal | 500mg/kg (500mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC BEHAVIORAL: MUSCLE WEAKNESS KIDNEY, URETER, AND BLADDER: RENAL FUNCTION TESTS DEPRESSED | Journal of Pharmacy and Pharmacology. Vol. 11, Pg. 150, 1959. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View