-
Name
Androsterone
- EINECS 200-173-4
- CAS No. 53-41-8
- Article Data109
- CAS DataBase
- Density 1.085 g/cm3
- Solubility 11.5mg/L(23.5 oC)
- Melting Point 181-184 °C(lit.)
- Formula C19H30O2
- Boiling Point 413.139 °C at 760 mmHg
- Molecular Weight 290.446
- Flash Point 176.377 °C
- Transport Information
- Appearance white to light beige crystalline powder
- Safety 22-24/25
- Risk Codes 36/37/38-43
-
Molecular Structure
- Hazard Symbols Xi
- Synonyms 3-Epihydroxyetioallocholan-17-one;3a-Hydroxy-17-androstanone;3a-Hydroxy-5a-androstan-17-one;3a-Hydroxy-5a-androstane-17-one;5a-Androstan-17-one-3a-ol;5a-Androstane-3a-ol-17-one;5a-Androsterone;Androkinin;Androtin;Atromide ICI;NSC9898;U 60366;
- PSA 37.30000
- LogP 3.95910
Synthetic route
-
-
111222-34-5
(3R,5S,8R,9S,10S,13S,14S)-10,13-Dimethyl-3-(tetrahydro-furan-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-one

-
-
53-41-8
cis-androsterone

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In ethanol for 1h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With methanol; sodium hydroxide at 40℃; for 4h; Inert atmosphere; | 94.6% |
| With potassium carbonate |

| Conditions | Yield |
|---|---|
| With Oxone; edetate disodium; sodium hydrogencarbonate In water; acetonitrile at 20℃; for 120h; | A 20% B 78% |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol; dichloromethane at 20℃; | 75% |

| Conditions | Yield |
|---|---|
| With trimethylamine-borane; silica gel; iron(III) chloride In benzene for 2h; Ambient temperature; | A 60.2% B 20.9% |
| With trimethylamine-borane; silica gel; iron(III) chloride In benzene for 2h; Product distribution; Ambient temperature; various catalysts and times; | A 60.2% B 20.9% |
| With hydrogen; Nic In tetrahydrofuran; ethanol at 25℃; under 760 Torr; for 1.16667h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |
-
-
5717-77-1, 15807-49-5, 35420-27-0, 57194-49-7
(3α,5α)-3-hydroxyandrostan-17-one 1,2-ethanediyl acetal

-
-
53-41-8
cis-androsterone

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In methanol; water for 0.25h; | 57% |
| With acetic acid |
-
-
592509-66-5
2-Oxo-heptanoic acid (3R,5S,8R,9S,10S,13S,14S)-10,13-dimethyl-17-oxo-hexadecahydro-cyclopenta[a]phenanthren-3-yl ester

-
A
-
53-41-8
cis-androsterone

| Conditions | Yield |
|---|---|
| With Oxone; edetate disodium; sodium hydrogencarbonate In water; acetonitrile at 20℃; for 72h; | A 45% B 55% |

| Conditions | Yield |
|---|---|
| With HEK-293 cells expressing Haliotis diversicolor supertexta 17β-hydroxysteroid dehydrogenase 11; fetal bovine serum In dimethyl sulfoxide for 23h; aq. buffer; Enzymatic reaction; | 53.4% |
| With rabbit 3-hydroxyhexobarbital dehydrogenase (AKR1C29); NADP In aq. phosphate buffer; ethyl acetate at 37℃; for 0.5h; pH=7.4; Catalytic behavior; Kinetics; Enzymatic reaction; | |
| With recombinant male golden hamster liver aldo-keto reductase AKR1C35; NAD In aq. phosphate buffer at 25℃; pH=7.4; Kinetics; Enzymatic reaction; |
-
-
10429-07-9
3β-tosyloxy-5α-androstan-17-one

-
A
-
53-41-8
cis-androsterone

-
B
-
846-46-8
androstanedione

-
C
-
14639-79-3, 24174-20-7, 82467-84-3, 963-75-7
5α-androst-2-en-17-one

| Conditions | Yield |
|---|---|
| With potassium nitrite In N,N-dimethyl-formamide at 80 - 85℃; for 18h; | A 51.5% B 0.03 g C 0.01 g |

-
-
846-46-8
androstanedione

-
A
-
481-29-8
Epiandrosterone

-
B
-
53-41-8
cis-androsterone

-
C
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| for 504h; Rhodotorula mucilaginosa; | A 18% B 35% C 30% |

-
-
521-18-6
Stanolone

-
A
-
481-29-8
Epiandrosterone

-
B
-
53-41-8
cis-androsterone

-
C
-
846-46-8
androstanedione

-
D
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| for 504h; Rhodotorula mucilaginosa; | A 21% B 33% C 5% D 33% |


| Conditions | Yield |
|---|---|
| With sodium nitrite In dimethyl sulfoxide at 90℃; for 29h; | A 29% B 29% C 31% |

-
-
1852-53-5
androstanediol

-
A
-
53-41-8
cis-androsterone

-
B
-
4363-08-0
(4aS,4bR,8R,10bR,12aS)-8-hydroxy-12a-methylhexadecahydro-2H-naphtho[2,1-f]chromen-2-one

| Conditions | Yield |
|---|---|
| With Aspergillus tamarii KITA (QM 1223) In N,N-dimethyl-formamide at 30℃; for 120h; Microbiological reaction; | A 23% B 29% |
-
-
58-22-0
testosterone

-
A
-
481-29-8
Epiandrosterone

-
B
-
63-05-8
Androstenedione

-
C
-
53-41-8
cis-androsterone

-
D
-
846-46-8
androstanedione

| Conditions | Yield |
|---|---|
| With Penicillium digitatum MRC 500787; MYB medium (malt extract 2percent, glucose 1percent, bacteriological peptone 1percent, yeast extract 0.3percent) In N,N-dimethyl-formamide at 24℃; for 120h; | A 8.9% B 16.3% C 3.6% D 6.4% |

-
-
53-43-0
dehydroepiandrosterone

-
A
-
53-42-9
Etiocholanolone

-
B
-
53-41-8
cis-androsterone


-
D
-
4150-30-5
5-androstene-3β,16α,17β-triol

| Conditions | Yield |
|---|---|
| aus dem Harn von Menschen nach der Injektion; |


| Conditions | Yield |
|---|---|
| With acetic acid; platinum at 20℃; Hydrogenation.zuletzt nach Zusatz von wenig Schwefelsaeure bei 70-75grad; | |
| With acetic acid; platinum at 20℃; Hydrogenation.zuletzt nach Zusatz von wenig Schwefelsaeure bei 70-75grad; |
-
-
20612-47-9
3β-chloro-5α-androstan-17-one

-
-
53-41-8
cis-androsterone

| Conditions | Yield |
|---|---|
| With potassium acetate; valeric acid anschliessend mit wss.-aethanol.NaOH; |
-
-
20612-47-9
3β-chloro-5α-androstan-17-one

-
A
-
53-41-8
cis-androsterone

-
B
-
14639-79-3, 24174-20-7, 82467-84-3, 963-75-7
5α-androst-2-en-17-one

| Conditions | Yield |
|---|---|
| With potassium acetate; acetic acid at 180℃; |
-
-
10429-07-9
3β-tosyloxy-5α-androstan-17-one

-
A
-
53-41-8
cis-androsterone

-
B
-
14639-79-3, 24174-20-7, 82467-84-3, 963-75-7
5α-androst-2-en-17-one

| Conditions | Yield |
|---|---|
| With aluminum oxide; Petroleum ether; benzene |

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide; acetic acid at 95℃; Erwaermen des aus dem Reaktionsgemisch mit Semicarbazid isol. 3α-Acetoxy-5α-androstanon-(17)-semicarbazons mit wss.Oxalsaeure und Erh. des erhalt. Oxalsaeure-bis-<17-oxo-5α-androstanyl-(3α)-esters> mit aethanol.Alkalilauge; | |
| With chromium(VI) oxide; acetic acid at 95℃; Erwaermen des aus dem Reaktionsgemisch mit Semicarbazid isol. 3α-Acetoxy-5α-androstanon-(17)-semicarbazons mit wss.Oxalsaeure und Erh. des erhalt. Oxalsaeure-bis-<17-oxo-5α-androstanyl-(3α)-esters> mit aethanol.Alkalilauge; |
-
-
143120-49-4
5α-stigmastanyl-(3α)-acetate

-
-
53-41-8
cis-androsterone

| Conditions | Yield |
|---|---|
| Gewinnung; |
-
-
20612-47-9
3β-chloro-5α-androstan-17-one

-
-
127-08-2
potassium acetate

-
-
64-19-7
acetic acid

-
A
-
53-41-8
cis-androsterone

-
B
-
14639-79-3, 24174-20-7, 82467-84-3, 963-75-7
5α-androst-2-en-17-one

| Conditions | Yield |
|---|---|
| at 180℃; |

-
-
10429-07-9
3β-tosyloxy-5α-androstan-17-one

-
-
127-08-2
potassium acetate

-
-
64-19-7
acetic acid

-
-
53-41-8
cis-androsterone

-
-
58-22-0
testosterone

-
A
-
481-29-8
Epiandrosterone

-
B
-
521-18-6
Stanolone

-
C
-
53-41-8
cis-androsterone

-
D
-
846-46-8
androstanedione

-
E
-
571-20-0
5-androgen-3,17-diol

-
F
-
1852-53-5
androstanediol

| Conditions | Yield |
|---|---|
| With total testicular homogenate of adult Sprague-Dawley rats treated with 6, des-Gly-NH210>LHRH ethylamide Product distribution; metabolism, <3H>labelled study, further: equine antibovine LH serum (JOAN-5-31-67); |

-
-
58-22-0
testosterone

-
A
-
521-18-6
Stanolone

-
B
-
53-41-8
cis-androsterone

-
C
-
846-46-8
androstanedione

-
D
-
571-20-0
5-androgen-3,17-diol

-
E
-
1852-53-5
androstanediol

| Conditions | Yield |
|---|---|
| With carbon dioxide; 5α-reductase in testicular cells of adult male Sprague-Dawley rats; oxygen; NADP at 37℃; for 1.5h; Product distribution; Kinetics; <3H>labelled, metabolism with or without 7α-hydroxytestosterone; |

-
-
63-05-8
Androstenedione

-
A
-
481-29-8
Epiandrosterone

-
B
-
53-41-8
cis-androsterone

-
C
-
846-46-8
androstanedione

-
D
-
62-84-0, 31427-20-0
7α-hydroxyandrost-4-ene-3,17-dione

-
E
-
571-20-0, 1851-23-6, 1852-53-5, 4069-06-1, 4069-07-2, 5856-10-0, 5856-11-1, 5864-32-4, 6038-31-9, 6165-21-5, 16989-87-0, 16989-88-1, 18485-82-0, 18485-84-2, 19767-69-2, 19905-98-7, 21862-73-7, 21862-74-8, 25126-76-5, 28980-34-9, 33526-31-7, 33526-32-8, 34864-27-2, 54324-44-6, 55821-63-1, 55821-64-2, 55821-65-3, 62356-64-3, 65451-37-8, 87099-87-4, 87099-93-2, 94667-48-8, 95975-22-7
5β-androstanediol

-
F
-
65451-37-8
5α-androstane-3α/β,17β-diol

| Conditions | Yield |
|---|---|
| With 7α-hydroxylase from microsomes of testicular tissue from Wistar rats injected or not intraperitoneally with human chorionic gonadotrophin till 24 h before kill; Krebs Ringer buffer; NADPH In ethanol at 37℃; for 0.25h; Product distribution; Kinetics; <4-14C>labeled study, Michaelis-Menton const. Km, maximal transformation rate Vm; |

-
-
63-05-8
Androstenedione

-
A
-
50-28-2
estradiol

-
B
-
53-16-7
Estrone

-
C
-
58-22-0
testosterone

-
D
-
521-18-6
Stanolone

-
E
-
53-42-9
Etiocholanolone

-
F
-
53-41-8
cis-androsterone

| Conditions | Yield |
|---|---|
| With carcinoma; gynecomastia; mammary dysplasia at 37℃; for 1.5h; Product distribution; cofactors under 95percent O2: 5percent CO2, <3H>labeled study; |


| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; divinylbenzene template polymer In tetrahydrofuran Product distribution; Ambient temperature; other steroid ketones; molecular imprinting of solid polymer; |


| Conditions | Yield |
|---|---|
| With potassium tri-sec-butyl-borohydride In tetrahydrofuran at -75℃; for 2h; | 63 % Turnov. |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 0.025h; microwave irradiation; | 100% |
| With pyridine at 20℃; | 100% |
| With dmap In dichloromethane at 20℃; | 97% |
-
-
53-41-8
cis-androsterone

-
-
2921-14-4, 7776-18-3, 20295-82-3
(aminooxy)acetic acid hemihydrochloride

| Conditions | Yield |
|---|---|
| With pyridine at 80℃; for 15h; | 97% |

| Conditions | Yield |
|---|---|
| With iron(II) chloride tetrahydrate; lithium In tetrahydrofuran; mineral oil at 20℃; for 4h; Reagent/catalyst; Inert atmosphere; stereoselective reaction; | 96% |
| With manganese(III) (Z)-2,2,6,6-tetramethyl-5-oxohept-3-en-3-olate; phenylsilane; oxygen In 1,2-dichloro-ethane; isopropyl alcohol at 23℃; under 760 Torr; | 47% |
| With methanol; sodium |

| Conditions | Yield |
|---|---|
| In acetonitrile for 2h; Substitution; Heating; | 96% |
-
-
53-41-8
cis-androsterone

-
-
107-30-2
chloromethyl methyl ether

-
-
1135993-39-3
(3R,5S,8R,9S,10S,13S,14S)-3-(methoxymethoxy)-10,13-dimethylHexadecahydro-17H-cyclopenta[a]phenanthren-17-one

| Conditions | Yield |
|---|---|
| With dmap; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 2h; | 95% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 16h; | 95% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 12.3h; | 77% |
-
-
53-41-8
cis-androsterone

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
57711-45-2
(3R,10S,13S)-3-((tert-butyldimethylsilyl)oxy)-10,13-dimethylhexadecahydro-17H-cyclopenta[a]phenanthren-17-one

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; | 94% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 93% |
| In methanol at 20℃; for 12h; | 86% |
| With 1H-imidazole In N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With pyridine at 20 - 25℃; for 4h; Inert atmosphere; | 92.2% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran for 5h; Wittig-Horner Reaction; | 92.1% |
-
-
53-41-8
cis-androsterone

-
-
4363-08-0
(4aS,4bR,8R,10bR,12aS)-8-hydroxy-12a-methylhexadecahydro-2H-naphtho[2,1-f]chromen-2-one

| Conditions | Yield |
|---|---|
| With calcium tetrakis(pentafluorophenyl)borate undecahydrate; dihydrogen peroxide; oxalic acid In 1,2-dichloro-ethane at 50℃; for 12h; Baeyer-Villiger Ketone Oxidation; regioselective reaction; | 92% |
-
-
53-41-8
cis-androsterone

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In methanol at 30℃; for 24h; Autoclave; | 92% |
| With ammonia; hydrogen In tetrahydrofuran at 120℃; for 15h; | 90% |
| With nickel(II) tetrafluoroborate hexahydrate; ammonia; hydrogen; bis(2-diphenylphosphinoethyl)phenylphosphine In 2,2,2-trifluoroethanol at 120℃; for 24h; chemoselective reaction; | 81% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran for 4h; Wittig Olefination; Reflux; | 91.2% |
| With potassium tert-butylate In tetrahydrofuran |
-
-
53-41-8
cis-androsterone

-
-
89685-22-3
5α-<16,16-2H2>androstane-3α-ol-17-one

| Conditions | Yield |
|---|---|
| With water-d2; sodium methylate In tetrahydrofuran; d(4)-methanol for 7h; Heating; | 91% |
| With deuteriated sodium hydroxide; deuteromethanol; water-d2 1) reflux, 48 h, 2) 4 deg C, overnight; Multistep reaction; |

| Conditions | Yield |
|---|---|
| In pyridine; N-methyl-acetamide; ethanol; water; ethyl acetate | 91% |


| Conditions | Yield |
|---|---|
| With 4-Benzoylpyridine In acetone at 20℃; for 23h; UV-irradiation; Inert atmosphere; | 90% |
| With perfluoro-cis-2-n-butyl-3-n-propyloxaziridine In various solvent(s) Ambient temperature; | 70% |
| With pyridinium chlorochromate |
-
-
913966-90-2
2-bromo-3,4,4-trichloro-3-butenoyl chloride

-
-
53-41-8
cis-androsterone

| Conditions | Yield |
|---|---|
| With pyridine In benzene at 20 - 23℃; | 90% |
-
-
53-41-8
cis-androsterone

-
-
151-50-8
potassium cyanide

-
-
100-46-9
benzylamine

-
-
125712-99-4
(3R,5S,8R,9S,10S,13S,14S)-17-Benzylamino-3-hydroxy-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthrene-17-carbonitrile

| Conditions | Yield |
|---|---|
| With acetic acid In methanol at 60℃; for 20h; | 89% |

-
-
53-41-8
cis-androsterone

| Conditions | Yield |
|---|---|
| With isopropyl alcohol; Ni(0) nanoparticles In tetrahydrofuran at 76℃; for 1h; | 89% |
-
-
53-41-8
cis-androsterone

-
-
80860-56-6
methyl uronate

-
-
80860-58-8, 80860-59-9
(2S,3S,4S,5R,6R)-3,4,5-Tris-benzyloxy-6-((3R,5S,8R,9S,10S,13S,14S)-10,13-dimethyl-17-oxo-hexadecahydro-cyclopenta[a]phenanthren-3-yloxy)-tetrahydro-pyran-2-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| boron trifluoride diethyl etherate In dichloromethane at -25℃; for 2h; | 88% |
-
-
53-41-8
cis-androsterone

-
-
59462-53-2
3α-hydroxy-16α-bromo-5α-androstan-17-one

| Conditions | Yield |
|---|---|
| With copper(I) bromide In methanol for 8h; Reflux; | 88% |
-
-
53-41-8
cis-androsterone

-
-
10481-80-8, 49566-77-0, 76023-63-7, 88930-99-8
androsterone hydrazone

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol for 1h; Heating; | 87% |
-
-
5397-03-5
hydrazinecarbodithioic acid methyl ester

-
-
53-41-8
cis-androsterone

-
-
1070968-28-3
C21H34N2OS2

| Conditions | Yield |
|---|---|
| In propan-1-ol at 20℃; for 2h; | 87% |
-
-
53-41-8
cis-androsterone

-
-
107-21-1
ethylene glycol

-
-
5717-77-1, 15807-49-5, 35420-27-0, 57194-49-7
(3α,5α)-3-hydroxyandrostan-17-one 1,2-ethanediyl acetal

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene Heating; | 85% |
| With toluene-4-sulfonic acid In toluene Reflux; | |
| With toluene-4-sulfonic acid In toluene Reflux; Dean-Stark; | |
| With toluene-4-sulfonic acid In toluene at 100℃; | |
| With toluene-4-sulfonic acid In toluene at 100℃; |
-
-
53-41-8
cis-androsterone

-
-
618-94-0
3-nitrobenzoic acid hydrazide

-
-
1197212-27-3
5α-androstan-3β-ol-17-one m-nitrobenzoylhydrazone

| Conditions | Yield |
|---|---|
| acetic acid In ethanol for 12h; Reflux; | 85% |
| With acetic acid In ethanol for 12h; Reflux; | 85% |
-
-
16717-64-9
1-azidostyrene

-
-
53-41-8
cis-androsterone

| Conditions | Yield |
|---|---|
| With 2,4,4,6-Tetrabromo-2,5-cyclohexadien-1-one In dichloromethane at 0 - 20℃; for 0.5h; Inert atmosphere; | 85% |
-
-
53-41-8
cis-androsterone

-
-
98-88-4
benzoyl chloride

-
-
5953-69-5
3α-hydroxy-5α-androstan-17-one 3-benzoate

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0 - 20℃; for 6h; | 85% |
-
-
53-41-8
cis-androsterone

-
-
14639-79-3, 24174-20-7, 82467-84-3, 963-75-7
5α-androst-2-en-17-one

| Conditions | Yield |
|---|---|
| With sulfuric acid; silica gel In toluene at 100℃; for 1h; | 83% |
| With diethylamino-sulfur trifluoride In dichloromethane at -78℃; for 1h; Inert atmosphere; regioselective reaction; | 30% |
| With boron trioxide at 300℃; |
-
-
53-41-8
cis-androsterone

-
-
109-94-4
formic acid ethyl ester

-
-
98391-23-2
(3α,5α)-3-hydroxy-16-hydroxymethyleneandrostan-17-one

| Conditions | Yield |
|---|---|
| With sodium methylate In toluene for 7h; Formylation; Heating; | 83% |

| Conditions | Yield |
|---|---|
| In propan-1-ol at 20℃; for 2h; | 82% |
Androsterone Chemical Properties
Chemical Formula: C19H30O2 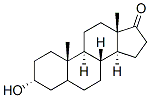
Molecular Weight: 290.44
Synonyms: 3-hydroxy-,(3alpha,5alpha)-androstan-17-on ; 5alpha-Androstan-17-one, 3alpha-hydroxy- ; 3-alpha-hydroxy-17-androstanone ; 3-Epihydroxyetioallocholan-17-one ; 5alpha-Androstane-3alpha-ol-17-one
Melting Point: 181-184 °C(lit.)
Flash Point: 176.4 °C
Boiling Point: 413.1 °C at 760 mmHg
Density of Androsterone (53-41-8) : 1.085 g/cm3
Vapour Pressure: 1.5E-08 mmHg at 25°C
alpha of Androsterone (53-41-8) : 96°(c=1, C2H5OH)
storage temp.: -20°C
Index of Refraction: 1.536
Chemical Properties: Androsterone (53-41-8) is a white to light beige crystalline powder
Androsterone Safety Profile
An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety Statements: 22: Do not breathe dust
24/25: Avoid contact with skin and eyes
WGK Germany: 3
RTECS: BV8053000
F: 3: Hygroscopic
21: Sensitive to humidity
StorageKeep container tightly closed. Keep container in a cool, well-ventilated area.
Androsterone Specification
Storage: Keep container in a cool, well-ventilated area. Keep container tightly closed.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View