-
Name
Aniline-2-sulfonic acid
- EINECS 201-810-9
- CAS No. 88-21-1
- Article Data79
- CAS DataBase
- Density 1.512 g/cm3
- Solubility sparingly soluble in water
- Melting Point 300 °C
- Formula C6H7NO3S
- Boiling Point 363oC
- Molecular Weight 173.192
- Flash Point
- Transport Information UN 2585 8/PG 3
- Appearance light brown powder
- Safety 26-36/37/39-45-28A
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Benzenesulfonicacid, o-amino- (6CI,7CI,8CI);1-Aminobenzene-2-sulfonic acid;2-Aminobenzenesulfonic acid;2-Aminobenzenesulphonic acid;2-Aminophenylsulfonic acid;2-Anilinesulfonic acid;2-Sulfoaniline;Benzenesulfonic acid,2-amino-;Aniline-o-sulphonic acid;NSC 147;Orthanilic acid;o-Aminobenzenesulfonic acid;o-Aminophenylsulfonic acid;o-Sulfanilic acid;
- PSA 88.77000
- LogP 2.17750
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydrogen sulfate; sulfuric acid at 200℃; for 19h; Reagent/catalyst; Temperature; | 99% |
| With chlorosulfonic acid; 1,1,2,2-tetrachloroethane |

| Conditions | Yield |
|---|---|
| With hydrogen at 120℃; under 30003 - 37503.8 Torr; for 0.75h; Reflux; | 97% |
| With Raney nickel at 90 - 120℃; under 6000.6 - 9750.98 Torr; | 4200 kg |

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid In 1,2-dichloro-benzene at 135℃; for 1h; Product distribution; Kinetics; Mechanism; E(activ), oth. temperatures; | A 3.2% B 96.8% |
| With sulfur trioxide In 1,2-dichloro-ethane at 5℃; Rate constant; Thermodynamic data; Product distribution; oth. temperature, E(activ.), var. ratios of reactants; | |
| With sulfuric acid In 1,2-dichloro-benzene at 180℃; Kinetics; Thermodynamic data; Equilibrium constant; variation of molar ratio and temperature, Ea, ΔGo, ΔHo, ΔSo; | |
| With sulfuric acid In 1,2-dichloro-benzene at 24.9℃; Thermodynamic data; Mechanism; Activation Free Energy, Enthalpy, Entropy of sulfonation and desulfonation; |

| Conditions | Yield |
|---|---|
| With (2,2':6',2''-terpyridine)(pyridine-2-carboxylate)iron(II)chloride; sodium hydroxide In water; acetonitrile | 95% |
-
-
4875-10-9
2-nitrothiophenol

-
A
-
1155-00-6
bis(2-nitrophenyl)disulfide

-
B
-
88-21-1
2-amino-1-benzenesulfonic acid

| Conditions | Yield |
|---|---|
| With water In 1,4-dioxane at 87℃; for 8h; | A 7% B 87% |
| In 1,4-dioxane; water at 87℃; for 6.25h; | A 20.4% B 16.7% |
| With water In 1,4-dioxane at 81 - 87℃; Rate constant; Mechanism; Product distribution; various water conc; different reaction times; |

| Conditions | Yield |
|---|---|
| With chloramine-B; sodium hydroxide; palladium dichloride In water; acetonitrile at 80℃; pH=12; | 85% |
| With [RuCl(P(C6H5)3)2(O(C6H4)NCH(C4H3N))]; sodium hydroxide In water; acetonitrile at 39.84℃; Kinetics; Concentration; Solvent; Temperature; |
-
-
1126-34-7
3-amino-benzenesulfonic acid, monosodium salt

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
62-53-3
aniline

-
C
-
121-57-3
4-aminobenzene sulfonic acid

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate; sodium dihydrogenphosphate In methanol for 0.0166667h; Product distribution; Mechanism; Irradiation; effect of solvent and pH; | A 15.6% B 37.2% C 39.3% |
| With perchloric acid; NaH2PO4-Na2HPO4 buffer; sodium chloride In water for 0.0166667h; Product distribution; Mechanism; Irradiation; | A 2.80 % Turnov. B 6.43 % Turnov. C 7.02 % Turnov. |


| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic acid |

| Conditions | Yield |
|---|---|
| With phosphorus; hydrogen iodide at 110℃; im Druckrohr; | |
| With sodium hydroxide; zinc |
-
-
154117-60-9
4-bromo-3-sulfo-benzoic acid

-
-
88-21-1
2-amino-1-benzenesulfonic acid

| Conditions | Yield |
|---|---|
| With ammonia at 180℃; im Rohr; |

| Conditions | Yield |
|---|---|
| With methanol | |
| Multi-step reaction with 2 steps 1: water; chlorine 2: natrium carbonate / Ansaeuern mit Essigsaeure und Kochen mit Eisenpulver View Scheme |

| Conditions | Yield |
|---|---|
| With ethanol; sulfur dioxide | |
| With sulfur dioxide; water |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite |

| Conditions | Yield |
|---|---|
| With sodium carbonate Ansaeuern mit Essigsaeure und Kochen mit Eisenpulver; | |
| With potassium hydroxide anschliessend Erwaermen mit N2H4+H2O und Palladium/Kohle oder Raney-Nickel; |

| Conditions | Yield |
|---|---|
| man reduziert das bei der Nitrierung entstandene Isomerengemisch nach Zugabe von Magnesiumoxyd mit Eisen und Schwefelsaeure; |
-
-
3768-55-6
N-trimethylsilylaniline

-
-
104184-87-4
chlorine ethoxysulfonate

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
3782-61-4
ethyl phenylamidosulfate

-
C
-
732944-81-9
N-ethyl-N-phenylamidosulfate

| Conditions | Yield |
|---|---|
| With acetic acid 1) CH2Cl2, (-10)-(-15) deg C, 2 h; reflux, 3 h; 2) (-10)-(-15) deg C, 3 h; Multistep reaction. Further byproducts given; |

-
-
2424-53-5
aniline sulfate

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
121-47-1
3-aminobenzenesulfonic acid

-
C
-
121-57-3
4-aminobenzene sulfonic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid at 40℃; Product distribution; Rate constant; Thermodynamic data; var. temperature, var. reaction time, var. ratio of reagents, presence of tetrabisulfatoboric acid, ΔE(activ.); | |
| With sulfuric acid at 180℃; for 7h; Thermodynamic data; Kinetics; Product distribution; ΔE(act.), various time, various temperature, various excess of H2SO4; | |
| With sulfur trioxide; mercury(II) sulfate at 7.6℃; for 0.5h; Thermodynamic data; Mechanism; Product distribution; E(activ.), various temperature, SO3 and HgSO4 concentration; |

-
-
2424-53-5
aniline sulfate

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
121-57-3
4-aminobenzene sulfonic acid

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-benzene at 180℃; for 0.5h; Rate constant; Thermodynamic data; Kinetics; other temperature (165 and 195 deg C), reaction time 7 h, activation energy; | A 3.9 % Spectr. B 96.1 % Spectr. |
| In 1,2-dichloro-benzene at 180℃; for 0.5h; Equilibrium constant; Mechanism; other temperature (165 and 195 deg C), reaction time 7 h; | A 3.9 % Spectr. B 96.1 % Spectr. |
| In 1,2-dichloro-benzene at 183℃; for 0.5h; Rate constant; Thermodynamic data; Kinetics; water removal, other solvent and temperature (p-chlorotoluene: 162 deg C, p-bromochlorobenzene: 196 deg C), reaction time 7 h; | A 3.8 % Spectr. B 96.2 % Spectr. |
-
-
15790-84-8
sodium phenylsulfamate

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
121-47-1
3-aminobenzenesulfonic acid

-
C
-
62-53-3
aniline

-
D
-
121-57-3
4-aminobenzene sulfonic acid

| Conditions | Yield |
|---|---|
| In methanol for 0.0166667h; Product distribution; Irradiation; | A 22.2 % Chromat. B 5.1 % Chromat. C 27.0 % Chromat. D 39.4 % Chromat. |
| In methanol Product distribution; Mechanism; Irradiation; varying solvents; | A 22.0 % Chromat. B 5.1 % Chromat. C 27.0 % Chromat. D 39.4 % Chromat. |

-
-
542-16-5, 2424-53-5, 20305-50-4, 29961-02-2
dianilunium sulphate

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
121-47-1
3-aminobenzenesulfonic acid

-
C
-
121-57-3
4-aminobenzene sulfonic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; mercury(II) sulfate at 60℃; for 1h; Title compound not separated from byproducts; | A 2.8 % Spectr. B 43.8 % Spectr. C 53.4 % Spectr. |
| With sulfuric acid; sulfur trioxide at 90℃; for 5h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With sulfuric acid at 90℃; for 5h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With sulfuric acid; mercury(II) sulfate at 60℃; for 1h; Product distribution; variation of reagent conc., time, temperature, and catalyst rate; | A 2.8 % Spectr. B 43.8 % Spectr. C 53.4 % Spectr. |

-
-
121-47-1
3-aminobenzenesulfonic acid

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
62-53-3
aniline

-
C
-
121-57-3
4-aminobenzene sulfonic acid

| Conditions | Yield |
|---|---|
| In methanol for 0.0166667h; Irradiation; | A 15.6 % Turnov. B 37.2 % Turnov. C 39.3 % Turnov. |

-
-
62-53-3
aniline

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
121-47-1
3-aminobenzenesulfonic acid

-
C
-
121-57-3
4-aminobenzene sulfonic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid at 180℃; for 7h; Yield given. Yields of byproduct given; | |
| With sulfuric acid; mercury(II) sulfate at 100℃; for 1h; Title compound not separated from byproducts; | A 2.4 % Spectr. B 32.0 % Spectr. C 65.6 % Spectr. |
| With sulfuric acid at 90℃; Kinetics; Rate constant; Thermodynamic data; other temp.(90 - 210 deg C); other conc.H2SO4 (80.06 - 99.71percent); E-activation; |


-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
137-51-9
4-aminobenzene-1,3-disulfonic acid

-
D
-
121-57-3
4-aminobenzene sulfonic acid

| Conditions | Yield |
|---|---|
| at 160℃; for 8h; Yield given. Yields of byproduct given; | |
| at 160℃; for 8h; Product distribution; Mechanism; other di- and trimethylanilinium butylamidosulfates, var. temperatures; |

-
-
121-57-3
4-aminobenzene sulfonic acid

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
121-47-1
3-aminobenzenesulfonic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid at 180℃; Title compound not separated from byproducts; | |
| With sulfuric acid at 180℃; Rate constant; Kinetics; other temperatures; other conc. of H2SO4; E(activ.); | |
| With sulfuric acid at 190℃; Product distribution; Rate constant; var. ratio of reactants, object of study - isomerization; | |
| With sulfuric acid at 180℃; Thermodynamic data; Rate constant; E(excit.); |
-
-
27489-27-6
<(2-Nitro-phenyl)sulfonyl>essigsaeure

-
-
88-21-1
2-amino-1-benzenesulfonic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
7732-18-5
water

-
-
62-53-3
aniline

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
3345-86-6
N-phenylsulfamic acid

-
-
60-29-7
diethyl ether

-
-
100-65-2
N-Phenylhydroxylamine

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
3345-86-6
N-phenylsulfamic acid

-
C
-
542-16-5, 2424-53-5, 20305-50-4, 29961-02-2
dianilunium sulphate

-
D
-
495-48-7
azoxybenzene

-
-
62-53-3
aniline

-
A
-
88-21-1
2-amino-1-benzenesulfonic acid

-
B
-
121-47-1
3-aminobenzenesulfonic acid

-
C
-
121-57-3
4-aminobenzene sulfonic acid

| Conditions | Yield |
|---|---|
| at 0 - 95℃; beim Behandeln von Anilin-sulfat; |

-
-
88-21-1
2-amino-1-benzenesulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-1-benzenesulfonic acid With sodium nitrite In water at 30℃; Stage #2: With sodium sulfite In water at 100 - 120℃; Stage #3: With sulfuric acid In water at 130℃; | 99.2% |
-
-
88-21-1
2-amino-1-benzenesulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-1-benzenesulfonic acid With sodium nitrite In water at 30℃; Acidic conditions; Flow reactor; Stage #2: With sodium sulfite In water at 95 - 110℃; Stage #3: With hydrogenchloride In water at 130℃; | 99.1% |

| Conditions | Yield |
|---|---|
| In water acid (2 mmol0 and Ag2O (1 mmol) stirred for 2 h; filtered, crystd. on slow evapn. overnight, elem. anal.; | 96% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-1-benzenesulfonic acid With sodium hydroxide; sodium nitrite In water at 0 - 5℃; for 0.0833333h; Stage #2: With hydrogenchloride In water at 0 - 5℃; for 0.5h; Stage #3: acetoacetamido With sodium acetate; sodium hydroxide In ethanol; water for 1h; | 92% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium carbonate; sodium nitrite In water at -10℃; for 0.5h; | 91% |
| With sodium hydroxide; sulfuric acid; sodium nitrite | |
| With ethanol; mixture of gaseous nitrogen oxides |
-
-
88-21-1
2-amino-1-benzenesulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-1-benzenesulfonic acid With toluene-4-sulfonic acid In acetonitrile for 0.166667h; Stage #2: With potassium iodide; sodium nitrite In water; acetonitrile at 10 - 20℃; for 1.16667h; | 91% |
| Stage #1: 2-amino-1-benzenesulfonic acid With hydrogenchloride; sodium nitrite In water at 0 - 5℃; for 0.5h; Stage #2: With sodium iodide In water at 0 - 50℃; for 14h; | 65% |
| Stage #1: 2-amino-1-benzenesulfonic acid With hydrogenchloride; sodium nitrite In water at 0℃; Stage #2: With sodium iodide In water at 0 - 50℃; for 14h; | 60% |
-
-
88-21-1
2-amino-1-benzenesulfonic acid

-
-
7087-68-5
N-ethyl-N,N-diisopropylamine

-
-
35037-73-1
1-isocyanato-4-trifluoromethoxy-benzene

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 8h; | 91% |

-
-
88-21-1
2-amino-1-benzenesulfonic acid

-
-
109-77-3
malononitrile

-
-
120814-56-4
2-(2-(dicyanomethylene)hydrazinyl)benzenesulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-1-benzenesulfonic acid With sodium hydroxide In water at 20℃; Stage #2: With hydrogenchloride; sodium nitrite In water at -0.16℃; for 1h; Stage #3: malononitrile With sodium acetate In ethanol; water at -0.16℃; for 1h; Japp-Klingemann reaction; | 90% |
-
-
88-21-1
2-amino-1-benzenesulfonic acid

-
-
104-12-1
p-chlorphenylisocyanate

-
-
7087-68-5
N-ethyl-N,N-diisopropylamine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 8h; | 89% |


| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 90℃; for 5h; pH=5 - 7; | 88% |
-
-
88-21-1
2-amino-1-benzenesulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-1-benzenesulfonic acid With sulfuric acid; sodium nitrite In water at 0℃; for 0.5h; Stage #2: With sodium azide In water at 20℃; for 15h; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-1-benzenesulfonic acid With hydrogenchloride; sodium hydroxide; sodium nitrite In water at 0 - 5℃; for 1h; Stage #2: Cyanoaceton With sodium acetate; sodium hydroxide In ethanol; water at 0℃; Japp-Klingemann Hydrazone Synthesis; | 86% |
-
-
37859-25-9
2-naphthylacetyl chloride

-
-
88-21-1
2-amino-1-benzenesulfonic acid

-
-
7087-68-5
N-ethyl-N,N-diisopropylamine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 4h; | 85% |

-
-
88-21-1
2-amino-1-benzenesulfonic acid

-
-
7087-68-5
N-ethyl-N,N-diisopropylamine

-
-
1548-13-6
p-trifluoromethyl-phenylisocyanate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 8h; | 85% |

-
-
88-21-1
2-amino-1-benzenesulfonic acid

-
-
34893-92-0
3,4-dichlorophenyl isocyanate

-
-
7087-68-5
N-ethyl-N,N-diisopropylamine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 8h; | 85% |

-
-
108-77-0
1,3,5-trichloro-2,4,6-triazine

-
-
88-21-1
2-amino-1-benzenesulfonic acid

-
-
90-20-0
4-amino-5-hydroxy-2,7-naphthalenedisulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-trichloro-2,4,6-triazine; 1-amino-2-sulfonic acid-4-(3-amino-2,4,6-trimethyl-5-sulfoamidophenyl)anthraquinone monosodium salt In water at 0 - 5℃; for 4h; pH=4- 6.5; Stage #2: 4-amino-5-hydroxy-2,7-naphthalenedisulfonic acid In water at 28 - 35℃; pH=3.5 - 4; Stage #3: 2-amino-1-benzenesulfonic acid Further stages; | 84.23% |

-
-
88-21-1
2-amino-1-benzenesulfonic acid

-
-
159596-05-1
4-morpholino-2-naphthol

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-1-benzenesulfonic acid With potassium carbonate In water Stage #2: With hydrogenchloride; sodium nitrite In water at 5 - 10℃; Stage #3: 4-morpholino-2-naphthol With sodium hydroxide In water for 0.5h; Cooling; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-1-benzenesulfonic acid With potassium carbonate In water Stage #2: With hydrogenchloride; sodium nitrite In water at 5℃; Stage #3: β-naphthol With sodium hydroxide In water for 0.5h; Cooling; | 84% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 4h; | 84% |
-
-
88-21-1
2-amino-1-benzenesulfonic acid

-
-
5416-93-3
4-Methoxyphenyl isocyanate

-
-
7087-68-5
N-ethyl-N,N-diisopropylamine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 8h; | 84% |

-
-
88-21-1
2-amino-1-benzenesulfonic acid

-
-
24677-78-9
2,3-dihydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With water In methanol at 20℃; for 24h; | 83% |
-
-
88-21-1
2-amino-1-benzenesulfonic acid

-
-
63059-25-6
2-iodobenzenesulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-1-benzenesulfonic acid With sodium carbonate In water Stage #2: With sodium nitrite In water at 0℃; for 0.75h; Further stages; | 82% |
| durch Diazotierung und Zersetzung der Diazoverbindung mit rauch.Jodwasserstoffsaeure; | |
| With sulfuric acid Diazotization.Erwaermen des Reaktionsprodukts mit Kaliumjodid; |

| Conditions | Yield |
|---|---|
| In ethanol; water at 20℃; for 24h; | 82% |
Aniline-2-sulfonic acid Chemical Properties
The molecular structure of Aniline-2-sulfonic acid (CAS NO.88-21-1):
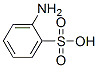
IUPAC Name: 2-Aminobenzenesulfonic acid
Molecular Weight: 173.18968 g/mol
Molecular Formula: C6H7NO3S
Density: 1.512 g/cm3
Melting Point: 300 °C
Index of Refraction: 1.629
Molar Refractivity: 40.71 cm3
Molar Volume: 114.4 cm3
Surface Tension: 67.5 dyne/cm
Water Solubility: sparingly soluble
XLogP3: 0.4
H-Bond Donor: 2
H-Bond Acceptor: 4
Rotatable Bond Count: 1
Exact Mass: 173.014664
MonoIsotopic Mass: 173.014664
Topological Polar Surface Area: 80.4
Heavy Atom Count: 11
Canonical SMILES: C1=CC=C(C(=C1)N)S(=O)(=O)O
InChI: InChI=1S/C6H7NO3S/c7-5-3-1-2-4-6(5)11(8,9)10/h1-4H,7H2,(H,8,9,10)
InChIKey: ZMCHBSMFKQYNKA-UHFFFAOYSA-N
EINECS: 201-810-9
Product Categories: Intermediates of Dyes and Pigments
Aniline-2-sulfonic acid Uses
Aniline-2-sulfonic acid (CAS NO.88-21-1) is used as dyes intermediates.
Aniline-2-sulfonic acid Toxicity Data With Reference
| 1. | mma-sat 6667 µg/plate | EMMUEG Environmental and Molecular Mutagenesis. 11 (Suppl 12)(1988),1. |
Aniline-2-sulfonic acid Consensus Reports
Reported in EPA TSCA Inventory.
Aniline-2-sulfonic acid Safety Profile
Hazard Codes:  C
C
Risk Statements: 34
R34:Causes burns.
Safety Statements: 26-36/37/39-45-28A
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S28:After contact with skin, wash immediately with plenty of soap-suds.
RIDADR: UN 2585 8/PG 3
WGK Germany: 3
RTECS: DB4727700
HazardClass: 8
PackingGroup: III
Aniline-2-sulfonic acid Specification
Aniline-2-sulfonic acid (CAS NO.88-21-1) is also named as 1-Aminobenzene-2-sulfonic acid ; 2-Aminobenzenesulfonic acid ; AI3-52212 ; Aniline-2-sulfonic acid ; Aniline-o-sulfonic acid ; Aniline-o-sulphonic acid ; Anilino-o-sulfonic acid ; Anilino-o-sulphonic acid ; CCRIS 4575 ; NSC 147 ; Orthanilic acid ; o-Aminobenzenesulfonic acid ; o-Aminophenylsulfonic acid ; o-Sulfanilic acid . Aniline-2-sulfonic acid (CAS NO.88-21-1) is light brown powder. It is soluble in water. Aniline-2-sulfonic acid is an amino acid. It reacts weakly with both acids and bases. Flash point data for Aniline-2-sulfonic acid are not available, however it is probably combustible.
Related Products
- Aniline-2-sulfonic acid
- 88214-48-6
- 88215-41-2
- 88-22-2
- 882238-14-4
- 88224-00-4
- 88224-02-6
- 88224-03-7
- 88224-05-9
- 882-26-8
- 88227-04-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View