-
Name
Azetidine
- EINECS 207-963-8
- CAS No. 503-29-7
- Article Data38
- CAS DataBase
- Density 0.851 g/cm3
- Solubility miscible in water
- Melting Point -70°C
- Formula C3H7N
- Boiling Point 64.9 °C at 760 mmHg
- Molecular Weight 57.0953
- Flash Point ?5°F
- Transport Information UN 2733 3/PG 2
- Appearance clear colorless liquid
- Safety 16-26-36/37/39-45
- Risk Codes 11-34
-
Molecular Structure
-
Hazard Symbols
 F,
F, C
C
- Synonyms 1,3-Propylenimine;Azacyclobutane;Azete, tetrahydro-;Trimethylenimine;
- PSA 12.03000
- LogP 0.30850
Synthetic route
-
-
78797-58-7, 78797-59-8, 78797-62-3, 36520-39-5
azetidine hydrochloride

-
-
503-29-7
azetidine

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 20 - 95℃; for 3h; Inert atmosphere; | 89% |
| With sodium hydroxide In dichloromethane; water | |
| With sodium hydroxide In water at 20℃; for 1h; | |
| With potassium hydroxide In water | |
| With sodium hydroxide In water at 20℃; for 1h; | 18 g |
-
-
78797-58-7, 78797-59-8, 78797-62-3, 36520-39-5
azetidine hydrochloride

-
-
503-29-7
azetidine

| Conditions | Yield |
|---|---|
| In water at 90℃; | 85% |
-
-
107128-00-7
N-benzhydryl azetidine

-
-
503-29-7
azetidine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium hydroxide; hydrogen; palladium on activated charcoal 1.) methanol, 60 deg C, 40 - 80 psi, 2 h, 2.) 100 deg C; | 83% |

-
-
503-29-7
azetidine

| Conditions | Yield |
|---|---|
| With sodium; ethylene glycol | 81% |

| Conditions | Yield |
|---|---|
| With triethylamine at 170℃; for 0.025h; microwave irradiation; | A n/a B 65% |

-
-
107128-00-7
N-benzhydryl azetidine

-
A
-
503-29-7
azetidine

-
B
-
54262-75-8
N-γ-aminopropyltrimethyleneimine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol other solvents; | A 50% B n/a |

| Conditions | Yield |
|---|---|
| Heating; | A 33% B 15% |
-
-
78064-88-7
3-<(Triphenylphosphoranylidene)amino>-1-propanol

-
-
503-29-7
azetidine

| Conditions | Yield |
|---|---|
| at 190℃; for 1.5h; | 29% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With pentan-1-ol; sodium at 120℃; | |
| With ammonia; sodium |

| Conditions | Yield |
|---|---|
| Destillation von salzsaurem Trimethylendiamin; |
-
-
14753-26-5
3-chloropropan-1-amine

-
A
-
503-29-7
azetidine

-
B
-
40226-15-1
3-(3-aminopropylamino)propan-1-ol

-
C
-
54262-75-8
N-γ-aminopropyltrimethyleneimine

-
D
-
129157-76-2
N1-(3-Azetidin-1-yl-propyl)-propane-1,3-diamine

-
E
-
107-11-9
1-amino-2-propene

-
F
-
156-87-6
propan-1-ol-3-amine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 80℃; Product distribution; | A n/a B 6 % Chromat. C 50 % Chromat. D 25 % Chromat. E n/a F 15 % Chromat. |


| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether at 0℃; |
-
-
14753-26-5
3-chloropropan-1-amine

-
-
107-15-3
ethylenediamine

-
A
-
503-29-7
azetidine

-
B
-
13531-52-7
N-(2-Aminoethyl)-1,3-propanediamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Product distribution; Heating; | A 3 % Chromat. B 96 % Chromat. |

-
-
14753-26-5
3-chloropropan-1-amine

-
-
107-15-3
ethylenediamine

-
A
-
503-29-7
azetidine

-
B
-
40226-15-1
3-(3-aminopropylamino)propan-1-ol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 50℃; Kinetics; Mechanism; Thermodynamic data; other temperatures; ΔH(excit.), ΔS(excit.), Ea; |


-
-
503-29-7
azetidine

| Conditions | Yield |
|---|---|
| With pentan-1-ol; sodium |

| Conditions | Yield |
|---|---|
| Kochen; |


| Conditions | Yield |
|---|---|
| Trockene Destillation; |

| Conditions | Yield |
|---|---|
| bei der Destillation; |

-
A
-
503-29-7
azetidine

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 80℃; |

| Conditions | Yield |
|---|---|
| at 25.5 - 35.5℃; Kinetik der Umwandlung; Reaktion des Hydrobromids; |

| Conditions | Yield |
|---|---|
| byproducts: NaCl; 0-20 ° C;; |


| Conditions | Yield |
|---|---|
| 0-20 ° C;; |


| Conditions | Yield |
|---|---|
| With dodecacarbonyl-triangulo-triruthenium; water; N–phenyl–2–(dicyclohexylphosphino)pyrrole In cyclohexane at 140℃; for 21h; Reagent/catalyst; Inert atmosphere; Schlenk technique; Autoclave; | |
| at 350℃; under 760.051 Torr; for 1h; Inert atmosphere; Flow reactor; |
-
-
503-29-7
azetidine

-
-
84358-13-4
N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid

-
-
957054-78-3
4-(azetidine-1-carbonyl)-piperidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 12h; | 100% |
| Stage #1: N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; triethylamine In tetrahydrofuran at 20℃; for 1h; Stage #2: azetidine With triethylamine In tetrahydrofuran for 14h; | 100% |
| Stage #1: N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; triethylamine In tetrahydrofuran at 22℃; for 1h; Stage #2: azetidine In tetrahydrofuran at 22℃; |
-
-
503-29-7
azetidine

-
-
1263868-24-1
2,4-dichloro-7-phenyl-6,7-dihydro-5H-cyclopenta[d]pyrimidine

-
-
1263868-28-5
4-(azetidin-1-yl)-2-chloro-7-phenyl-6,7-dihydro-5H-cyclopenta[d]pyrimidine

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 0.5h; | 100% |
| In methanol at 20℃; for 0.5h; | 100% |
-
-
503-29-7
azetidine

-
-
1380329-77-0
1-methyl-5-(2-phenyl-[1,2,4]triazolo[1,5-a]pyridin-7-ylcarbamoyl)-1H-pyrazole-4-carboxylic acid

-
-
1380329-78-1
4-(azetidine-1-carbonyl)-2-methyl-2H-pyrazole-3-carboxylic acid (2-phenyl-[1,2,4]triazolo[1,5-a]pyridin-7-yl)amide

| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; ethyl acetate at 70℃; for 2h; | 100% |
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; ethyl acetate at 70℃; for 2h; | 100% |
-
-
503-29-7
azetidine

-
-
1443432-71-0
N-{1-[N-(benzyloxycarbonyl)-sulfamoyl]pyridin-4(1H)-ylidene}-N-methylmethanaminium chloride

-
-
1271835-78-9
benzyl (azetidin-1-ylsulfonyl)carbamate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 100% |
| In dichloromethane at 20℃; | 100% |
-
-
503-29-7
azetidine

-
-
1616070-35-9
3-chloro-5-(3-iodo-1H-pyrazol-1-yl)pyridazine

-
-
1621525-33-4
3-(azetidin-1-yl)-5-(3-iodo-1H-pyrazol-1-yl)pyridazine

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 60℃; for 6h; | 100% |

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 120℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: C69H116N6O29 With N-ethyl-N,N-diisopropylamine; O‐(1H‐benzotriazol‐1‐yl)‐N,N,N′,N′‐tetramethyluronium tetrafluoroborate In N,N-dimethyl-formamide at 0 - 20℃; for 3h; Stage #2: azetidine at 55℃; Sealed tube; | 100% |
| Stage #1: C69H116N6O29 With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; for 3h; Stage #2: azetidine In N,N-dimethyl-formamide at 55℃; Sealed tube; | 100% |
-
-
503-29-7
azetidine

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate In toluene at 110℃; for 24h; Buchwald-Hartwig Coupling; Sealed tube; Inert atmosphere; | 100% |
-
-
503-29-7
azetidine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.166667h; | 100% |
-
-
503-29-7
azetidine

-
-
41071-36-7
(E)-O-methyl-4-nitrobenzohydroximoyl chloride

-
-
142701-85-7
Azetidin-1-yl-(4-nitro-phenyl)-methanone O-methyl-oxime

| Conditions | Yield |
|---|---|
| In benzene at 32℃; | 99.7% |
| In benzene at 32℃; Rate constant; | |
| In acetonitrile at 32℃; Rate constant; different amine excess; | |
| In acetonitrile at 32℃; |
-
-
503-29-7
azetidine

-
-
32115-53-0
N-chloroazetidine

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide at 20℃; under 0.1 Torr; | 99% |
| With N-chloro-succinimide under 0.001 Torr; for 3h; Ambient temperature; | |
| With N-chloro-succinimide; chlorine at 600℃; quarz U-tube; | |
| With N-chloro-succinimide | |
| With N-chloro-succinimide at 46.9℃; under 0.008 - 0.08 Torr; Yield given; |
-
-
503-29-7
azetidine

-
-
188699-17-4
5-bromomethyl-2,3-dimethoxy-7-nitro-quinoxaline

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydroxide In dichloromethane | 99% |
-
-
503-29-7
azetidine

-
-
920490-05-7
6-(2-hydroxyethyl)-2-(methylthio)-4-pyrimidinyl 4-methyl-1-benzenesulfonate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 70℃; for 48h; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 90℃; for 3h; Inert atmosphere; | 99% |
| With PS-morpholine In chloroform at 50℃; for 2h; |
-
-
503-29-7
azetidine

-
-
1227210-47-0
5-(bromomethyl)-1-methyl-3-nitro-1H-pyrazole

-
-
1227210-48-1
5-(azetidin-1-ylmethyl)-1-methyl-3-nitro-1H-pyrazole

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; | 99% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; for 24h; | 90% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; for 24h; | 90% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; for 24h; | 75.1% |
-
-
503-29-7
azetidine

-
-
1174886-97-5
5-(ethoxycarbonyl)-1-methyl-1H-pyrazole-4-carboxylic acid

-
-
1278407-69-4
4-(azetidine-1-carbonyl)-2-methyl-2H-pyrazole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With propylphosphonic anhydride; N-ethyl-N,N-diisopropylamine In ethyl acetate at 0 - 20℃; | 99% |
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; ethyl acetate at 0 - 20℃; | 60.7% |
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In ethyl acetate at 0 - 20℃; | |
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In ethyl acetate at 20℃; for 3h; Cooling; |
-
-
503-29-7
azetidine

-
-
36953-42-1
3-bromo-4-chloropyridine

-
-
1289267-06-6
4-(azetidin-1-yl)-3-bromopyridine

| Conditions | Yield |
|---|---|
| With caesium carbonate In 1,2-dimethoxyethane at 90℃; for 18h; Inert atmosphere; capped; | 99% |
| With caesium carbonate In 1,2-dimethoxyethane at 90℃; for 18h; Inert atmosphere; | 66% |
-
-
503-29-7
azetidine

-
-
1421787-27-0
(E)-2-{8-[(4-chloro-2-methyl-6-propylpyrimidin-5-yl)methyl]-3-fluorodibenzo[b,e]oxepin-11(6H)-ylidene}propanenitrile

-
-
1421787-24-7
(E)-2-(8-{[4-(azetidin-1-yl)-2-methyl-6-propylpyrimidin-5-yl]methyl}-3-fluorodibenzo[b,e]oxepin-11(6H)-ylidene)propanenitrile

| Conditions | Yield |
|---|---|
| In ethanol at 50℃; for 2h; | 99% |

| Conditions | Yield |
|---|---|
| at 35℃; for 18h; | 99% |
-
-
503-29-7
azetidine

| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In dichloromethane; N,N-dimethyl-formamide | 99% |
-
-
503-29-7
azetidine

| Conditions | Yield |
|---|---|
| With triethylamine In dimethyl sulfoxide at 80℃; for 0.0833333h; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran 1.) 0 deg C, 20 min, 2.) RT, 1 h; | 98% |

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.666667h; | 98% |
| In dichloromethane for 0.666667h; | 98% |
| In dichloromethane for 1h; | 97% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran; dichloromethane at 0 - 20℃; Inert atmosphere; | 98% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran; chloroform at 0℃; for 0.333333h; |

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.666667h; | 98% |
-
-
503-29-7
azetidine

-
-
38883-39-5
cis-Cl2(Ph3P)Pd(CNC6H4-p-OMe)

| Conditions | Yield |
|---|---|
| In tetrahydrofuran To suspn. of Pd compd. is added azetidine, then react. mixture is stirred at 0°C, is allowed to warm to room temp., mixture is stirred at room temp. for 10 h (under N2).; monitored by IR, ppt. is filtered off, washed with n-hexane, dried under vacuum, recrystd. from CH2Cl2-n-hexane, elem. anal.; | 98% |
-
-
503-29-7
azetidine

-
-
4746-97-8
cyclohexanedione monoethylene ketal

-
-
151-50-8
potassium cyanide

-
-
1002916-64-4
8-azetidin-1-yl-1,4-dioxaspiro[4,5]decane-8-carbonitrile

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 0 - 20℃; for 120h; | 98% |

-
-
503-29-7
azetidine

-
-
4746-97-8
cyclohexanedione monoethylene ketal

-
-
1002916-64-4
8-azetidin-1-yl-1,4-dioxaspiro[4,5]decane-8-carbonitrile

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 20℃; for 120h; Cooling with ice; | 98% |

-
-
503-29-7
azetidine

-
-
74386-55-3
5-phenylmethoxypyridin-2-carboxylic acid

-
-
1208536-50-8
2-(azetidin-1-ylcarbonyl)-5-(benzyloxy)pyridine

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In N,N-dimethyl-formamide at 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 70℃; for 1h; | 98% |
Azetidine Specification
The Azetidine, with the CAS registry number 503-29-7 and EINECS registry number 207-963-8, is a heterocyclic organic compound. And the molecular formula of this chemical is C3H7N. It is a kind of clear colorless liquid, and belongs to the class of four membered rings and it contains a nitrogen atom. It is sensitive to moisture and air, and should be stored at 2-8°C with the shielding gas argon.
The physical properties of Azetidine are as following: (1)ACD/LogP: -0.20; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -3.3; (4)ACD/LogD (pH 7.4): -3.23; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 3.24 Å2; (13)Index of Refraction: 1.426; (14)Molar Refractivity: 17.18 cm3; (15)Molar Volume: 67 cm3; (16)Polarizability: 6.81×10-24cm3; (17)Surface Tension: 27.1 dyne/cm; (18)Density: 0.851 g/cm3; (19)Enthalpy of Vaporization: 30.69 kJ/mol; (20)Boiling Point: 64.9 °C at 760 mmHg; (21)Vapour Pressure: 162 mmHg at 25°C.
Uses of Azetidine: It can react with bis-(3-amino-propyl)-amine to produce N,N'-bis-(3-amino-propyl)-propanediyldiamine. This reaction will need catalyst palladium black. The reaction time is 12 hours with temperature of 120°C, and the yield is about 75%.
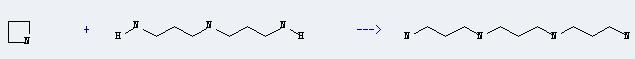
You should be cautious while dealing with this chemical. It is a kind of highly flammale chemical which may cause burns. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; Keep away from sources of ignition - No smoking; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: N1CCC1
(2)InChI: InChI=1/C3H7N/c1-2-4-3-1/h4H,1-3H2
(3)InChIKey: HONIICLYMWZJFZ-UHFFFAOYAE
Related Products
- Azetidine
- Azetidine hydrochloride
- Azetidine, 1-[(4-bromophenyl)sulfonyl]-
- Azetidine, 2-phenyl-
- Azetidine, 3-(3-fluorophenoxy)-
- Azetidine, 3-bromo-,hydrochloride (1:1)
- Azetidine, 3-fluoro-
- Azetidine,1-(2,6-dimethylphenyl)-
- Azetidine,1-(diphenylmethyl)-3-iodo-
- Azetidine,3-(4-fluorophenoxy)-
- 503-30-0
- 503300-35-4
- 503309-11-3
- 50332-66-6
- 5033-28-3
- 50335-03-0
- 503-38-8
- 50-33-9
- 50-34-0
- 503-40-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View