-
Name
Benzyl bromide
- EINECS 202-847-3
- CAS No. 100-39-0
- Article Data503
- CAS DataBase
- Density 1.438 g/cm3
- Solubility
- Melting Point -3 ºC
- Formula C7H7Br
- Boiling Point 198.499 ºC at 760 mmHg
- Molecular Weight 171.037
- Flash Point 86.667 ºC
- Transport Information UN 1737 6.1/PG 2
- Appearance clear light amber liquid
- Safety 39-2
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Toluene,a-bromo- (8CI);(Bromomethyl)benzene;(Bromophenyl)methane;1-Bromomethylbenzene;NSC 8041;Phenylmethyl bromide;a-Bromotoluene;w-Bromotoluene;
- PSA 0.00000
- LogP 2.58150
Synthetic route

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane for 1.5h; Ambient temperature; | 100% |
| With manganese(IV) oxide; bromine In dichloromethane at 0℃; for 1h; Product distribution; Further Variations:; Solvents; Temperatures; reaction time; | 100% |
| With bromine; sodium t-butanolate In cyclohexane Heating; | 100% |

| Conditions | Yield |
|---|---|
| With 1,1,1,2,2,2-hexamethyldisilane; pyridinium hydrobromide perbromide In chloroform at 25℃; for 0.5h; | 100% |
| With phosphorus tribromide In benzene at 20℃; | 100% |
| Stage #1: benzyl alcohol With 1,2,3-Benzotriazole; thionyl chloride In dichloromethane for 0.0833333h; Stage #2: With potassium bromide In dichloromethane; N,N-dimethyl-formamide for 0.5h; | 99% |

-
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| With bromine In acetonitrile one-electron oxidn. of cis-Co complex by Br2 at 298 K; monitored by (1)H-NMR; | 100% |
-
-
103514-63-2
C13H25N3PS(1+)

-
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| With bromide In N,N-dimethyl-formamide | 98% |

| Conditions | Yield |
|---|---|
| With Dichloromethylsilane; phosphorus tribromide; iron(III) chloride In acetonitrile for 4h; Heating; | 97% |
| With polymethylhydrosiloxane; dimethylbromosulphonium bromide In chloroform at 20℃; for 5h; | 96% |
| With chloro-trimethyl-silane; 1,1,3,3-Tetramethyldisiloxane; lithium bromide In acetonitrile at 80℃; for 30h; | 94% |

| Conditions | Yield |
|---|---|
| With bismuth(III) bromide In 1,2-dichloro-ethane at 25℃; for 2.25h; | 97% |
| With pyridine; tributyltin bromide at 50℃; Thermodynamic data; Equilibrium constant; other temperatures, Δ G; | 26 % Spectr. |

| Conditions | Yield |
|---|---|
| With triethylsilane; Acetyl bromide; tin(II) bromide In dichloromethane for 3h; Ambient temperature; | 96% |

| Conditions | Yield |
|---|---|
| With 4-aminophenyl diphenylphosphinite; N-Bromosuccinimide In dichloromethane at 20℃; for 2h; | 95% |
| With boron trifluoride diethyl etherate; lithium bromide In acetonitrile for 24h; Ambient temperature; | 89% |
| With 1-(2-OPPh2-propyl)-3-methylimidazolium hexafluorophosphate; bromine at 80℃; for 2h; | 89% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide at 130℃; for 1h; | 95% |
| Multi-step reaction with 2 steps 1: 12 N HCl / 105 °C 2: 93 percent / 47percent aq. HBr / 1 h / 130 °C View Scheme |
-
-
14642-79-6
benzyloxy-trimethylsilane

-
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| With 4-aminophenyl diphenylphosphinite; bromine In dichloromethane at 20℃; | 95% |
| With 1-(2-OPPh2-propyl)-3-methylimidazolium hexafluorophosphate; bromine at 80℃; for 1h; | 87% |
| With Tri-n-butylfluorphosphoniumbromid In benzene at 20℃; for 24h; | 81% |
-
-
55791-06-5
benzyl mesylate

-
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| With 1-n-butyl-3-methylimidazolim bromide at 50℃; for 1h; Inert atmosphere; Green chemistry; | 95% |
| With lithium bromide In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; | 89% |
| With calcium bromide In dimethyl sulfoxide for 3h; Ambient temperature; | 79% |
-
-
35052-27-8
3,5-dimethoxy-4-methylphenylmethyl alcohol

-
A
-
35047-51-9
5-(bromomethyl)-1,3-dimethoxy-2-methylbenzene

-
B
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| With phosphorus tribromide | A n/a B 95% |

| Conditions | Yield |
|---|---|
| With water; boron tribromide In chloroform-d1 at 20℃; Solvent; | A 95% B 88% |

| Conditions | Yield |
|---|---|
| With calcium bromide; tetrahexylammonium bromide at 110℃; for 24h; | 94% |
| With hydrogen bromide at 130℃; for 1h; | 93% |
| With 1-bromo-butane; Mg6Al2(OH)16Cl2*4H2O In N,N-dimethyl-formamide at 69.9℃; for 20h; | 90% |

| Conditions | Yield |
|---|---|
| With Silphos; bromine In acetonitrile for 0.166667h; Heating; | 92% |
| With N-Bromosuccinimide; triphenylphosphine In dichloromethane at 20℃; for 5h; | 72% |

| Conditions | Yield |
|---|---|
| With triethylsilane; Acetyl bromide; tin(II) bromide In dichloromethane for 3h; Ambient temperature; | 91% |
-
-
53172-91-1
(benzyloxy)(tert-butyl)dimethylsilane

-
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane for 0.166667h; Ambient temperature; | 90% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; hydrogen bromide; potassium bromide In water at 20℃; Irradiation; Green chemistry; | A 90% B n/a |
| With N-hydroxyphthalimide; carbon tetrabromide; trichloroisocyanuric acid; cobalt(II) diacetate tetrahydrate In dichloromethane at 25℃; for 20h; |

| Conditions | Yield |
|---|---|
| With tetra-N-butylammonium tribromide; dibromoisocyanuric acid In dichloromethane at 20℃; for 2h; UV-irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With triethylsilane; Acetyl bromide; tin(II) bromide In dichloromethane for 3h; Ambient temperature; | 89% |
-
-
631-61-8
ammonium acetate

-
-
100-51-6
benzyl alcohol

-
A
-
140-11-4
Benzyl acetate

-
B
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; triphenylphosphine In acetonitrile at 20℃; for 2h; Cooling with ice; | A 87% B 3% |
| With bromine; triphenylphosphine In acetonitrile at 20℃; for 0.7h; | A 40% B 53% |


| Conditions | Yield |
|---|---|
| With bromine In dichloromethane for 2h; Ambient temperature; Irradiation; | A 11% B 86% |
| With hydrogen bromide; dihydrogen peroxide In water at 20℃; for 10h; Irradiation; | A 4% B 81% |
| With hydrogen bromide; dihydrogen peroxide In water at 27℃; for 24h; Irradiation; | A 7% B 81% |

-
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| Stage #1: 4-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-ethyl-piperidine-1-carboxylic acid tert-butyl ester With hydrogenchloride In ethyl acetate for 4.08333h; Stage #2: With water; sodium hydrogencarbonate In ethyl acetate | 84% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In dichloromethane; benzene Irradiation (UV/VIS); nitrogen atmosphere; mercurial and NBS (2.5 equiv)/CH2Cl2 in deoxygenated solvent irradiated (250-W sunlamp, 40 °C, 6h);; filtration; solvent removed (vacuum); PhCH2CH2Ph and PhCH2Br analysed by (1)H NMR, GLC and GCMS;; | A 84% B 4% |
| With N-Bromosuccinimide In dichloromethane; benzene Irradiation (UV/VIS); nitrogen atmosphere; mercurial and NBS (2.5 equiv)/CH2Cl2 in deoxygenated solvent irradiated (250-W sunlamp, 40 °C, 4h) in the presence of 20 mol% (t-Bu)2NO (radical);; filtration; solvent removed (vacuum); PhCH2CH2Ph and PhCH2Br analysed by (1)H NMR, GLC and GCMS;; | A 46% B 2% |
-
-
64-19-7
acetic acid

-
-
108-88-3
toluene

-
A
-
100-39-0
benzyl bromide

-
B
-
98-86-2
acetophenone

-
C
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With hydrogen bromide; oxygen; bromide; cobalt(II) acetate; manganese(II) acetate at 75℃; Kinetics; Product distribution; atmospheric pressure; | A n/a B n/a C 83% |

-
-
116670-02-1
{(η5-C5Me5)Os(CO)(PMe2Ph)CH2Ph}

-
-
7726-95-6
bromine

-
A
-
107087-80-9
{(η5-C5Me5)Os(CO)(PMe2Ph)Br}

-
B
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 (N2); electrophilic cleavage reaction by addn. of bromine to a soln. of Cp*Os(CO)(PMe2Ph)CH2Ph in CD2Cl2;; not isolated, detected by NMR;; | A 77% B 83% |

-
-
54673-14-2
[(ethoxymethoxy)methyl]benzene

-
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; 1-(n-butyl)-3-methylimidazolium tetrachloroindate at 135 - 140℃; for 0.075h; Microwave irradiation; Neat (no solvent); chemoselective reaction; | 83% |
-
-
1539-57-7
diethyl 2,6-dimethyl-4-benzyl-1,4-dihydropyridine-3,5-dicarboxylate

-
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; tris(2,2′-bipyridyl)dichlororuthenium(II) hexahydrate; sodium bromide In water; acetonitrile at 20℃; for 24h; Sealed tube; Inert atmosphere; Irradiation; regioselective reaction; | 83% |

| Conditions | Yield |
|---|---|
| 100% | |
| In acetone; benzene at 20℃; for 24h; | 100% |
| In dichloromethane at 20℃; for 12h; | 100% |
-
-
616-47-7
1-methyl-1H-imidazole

-
-
100-39-0
benzyl bromide

-
-
65039-11-4
3-benzyl-1-methylimidazolium bromide

| Conditions | Yield |
|---|---|
| at 100℃; for 2h; | 100% |
| In 1,4-dioxane at 100℃; for 12h; | 98% |
| In ethanol; acetonitrile at 80℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| In methanol for 336000h; Heating; | 100% |
| In acetone; benzene at 20℃; for 24h; | 100% |
| In methanol for 336h; Heating; | 100% |
-
-
98-92-0
nicotinamide

-
-
100-39-0
benzyl bromide

-
-
13076-43-2
3-(aminocarbonyl)-1-benzylpyridinium bromide

| Conditions | Yield |
|---|---|
| In acetone for 12h; Reflux; | 100% |
| In acetonitrile | 93% |
| In acetonitrile at 140℃; for 1.5h; Sealed tube; Microwave irradiation; | 89% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetone Heating; | 100% |
| With caesium carbonate In acetonitrile at 50℃; | 98% |
| With caesium carbonate In acetonitrile at 50℃; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 6h; | 100% |
| Stage #1: 4-bromo-phenol With sodium hydride In N,N-dimethyl-formamide at 5℃; for 0.333333h; Inert atmosphere; Stage #2: benzyl bromide In N,N-dimethyl-formamide at 20℃; for 2h; Inert atmosphere; | 99% |
| With potassium phosphate; tetrabutylammomium bromide In water at 20℃; for 2h; Sealed tube; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| In toluene at 95℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 3h; | 100% |
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 3h; | 87% |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 12h; Inert atmosphere; | 48.6% |
-
-
4920-77-8
3-methyl-2-nitrophenol

-
-
100-39-0
benzyl bromide

-
-
61535-21-5
1-(benzyloxy)-3-methyl-2-nitrobenzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Heating / reflux; | 100% |
| With potassium carbonate In acetonitrile at 40 - 75℃; for 7.75h; | 99% |
| With potassium carbonate; acetone |
-
-
3943-97-3
methyl 4-hydroxycinnamate

-
-
100-39-0
benzyl bromide

-
-
84184-51-0
(E)-methyl 3-(4-(benzyloxy)phenyl)acrylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 23℃; for 4h; Reflux; Inert atmosphere; | 100% |
| With sodium hydride In N,N-dimethyl-formamide at 20℃; for 14h; Etherification; | 87% |
| With sodium hydroxide In ethanol | 82% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 1.16667h; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; | 100% |
| With potassium carbonate In acetonitrile for 3h; | 100% |

| Conditions | Yield |
|---|---|
| In xylene for 0.00833333h; Heating; microwave irradiation; | 100% |
| In toluene for 12h; Reflux; | 100% |
| at 100℃; for 0.0333333h; Irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Heating; | 100% |
| With caesium carbonate In acetone at 20℃; | 100% |
| With potassium carbonate In acetonitrile at 20℃; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,4-Dihydroxybenzaldehyde With sodium hydrogencarbonate In acetonitrile at 20℃; for 0.75h; Inert atmosphere; Stage #2: benzyl bromide In acetonitrile Inert atmosphere; Reflux; | 100% |
| With potassium hydrogencarbonate In acetonitrile for 15h; Reflux; | 85% |
| With sodium hydrogencarbonate In acetonitrile for 15h; Reflux; | 85% |

| Conditions | Yield |
|---|---|
| With TiCl2*2THF In tetrahydrofuran for 24h; Heating; | 100% |
| With Wilkinson's catalyst; dimethyl zinc(II) In tetrahydrofuran; hexane at 20℃; for 1h; | 100% |
| With hafnium(II) chloride In tetrahydrofuran for 12h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With sodium iodide In acetone at 20℃; for 24h; | 100% |
| With sodium iodide In acetone at 20℃; for 24h; Inert atmosphere; Darkness; | 100% |
| With hydrogen iodide; cetyltributylphosphonium bromide at 60℃; for 0.0833333h; | 99% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 75℃; | 100% |
| Inert atmosphere; Neat (no solvent); | 99% |
| for 5h; Reflux; | 93% |
-
-
2973-76-4
5-bromo-4-hydroxy-3-methoxybenzaldehyde

-
-
100-39-0
benzyl bromide

-
-
2556-04-9
4-(benzyloxy)-3-bromo-5-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 4h; | 97% |
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; | 94% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 100% |
| With potassium carbonate In acetone for 8h; Heating; | 97% |
| With potassium carbonate In acetone for 8h; Heating / reflux; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: isopropyl alcohol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.5h; Inert atmosphere; Stage #2: benzyl bromide In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; for 5h; Inert atmosphere; | 100% |
| With iron(II) sulfate at 75℃; for 12h; | 92% |
| With silver(II) oxide Heating; | 90% |
| With potassium hydroxide; tetrafluoroboric acid; mercury(II) oxide In dichloromethane 1.) room temp., 1 h; | 60% |
| (i) Ag2O, (ii) Py, PhCOCl; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With potassium carbonate; tetrabutylammomium bromide for 96h; Ambient temperature; | 100% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide at 80℃; for 2h; | 91% |
| With 1-butyl-3-methylimidazolium hydroxide at 100℃; under 51.7148 Torr; for 0.0833333h; microwave irradiation; | 82% |

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; sodium hydride In trichlorophosphate Heating; | 100% |
| With tetra-(n-butyl)ammonium iodide; sodium hydride In tetrahydrofuran at 23℃; for 5.5h; | 100% |
| With tetra-(n-butyl)ammonium iodide; sodium hydride In tetrahydrofuran; mineral oil at 0 - 23℃; for 18h; Inert atmosphere; Sealed tube; | 100% |
-
-
100-39-0
benzyl bromide

-
-
15285-42-4
benzyl nitrate

| Conditions | Yield |
|---|---|
| With silver nitrate In acetonitrile at 70℃; for 5h; Darkness; | 100% |
| With silver nitrate In acetonitrile at 20℃; Nitration; | 97% |
| With silver nitrate In acetonitrile at 20℃; for 24h; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: 1H-imidazole With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 2h; Stage #2: benzyl bromide In N,N-dimethyl-formamide at 20℃; for 4h; | 100% |
| Stage #1: 1H-imidazole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.25h; Stage #2: benzyl bromide In N,N-dimethyl-formamide; mineral oil at 25℃; for 3h; | 97% |
| With caesium carbonate at 100℃; for 0.416667h; Microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| 100% | |
| In acetone Reflux; | 100% |
| In toluene for 14h; Inert atmosphere; Reflux; | 72% |

| Conditions | Yield |
|---|---|
| 100% | |
| In acetonitrile at 45℃; for 3h; | 99% |
| In acetonitrile at 100℃; for 12h; | 98.71% |

| Conditions | Yield |
|---|---|
| 100% | |
| at 0 - 20℃; | 99.6% |
| at 0 - 20℃; | 99.6% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 4h; 0 deg C to 25 deg C; | 100% |
| Stage #1: caprolactam With sodium hydride In tetrahydrofuran at 0℃; for 0.5h; Inert atmosphere; Stage #2: benzyl bromide In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 98% |
| With sodium hydride In tetrahydrofuran; paraffin 1.) 0 deg C to room temperature, 2 h, 2.) room temperature, 2 h; | 96% |
Benzyl bromide Specification
The Benzyl bromide, with the CAS registry number 100-39-0, is also known as a-Bromotoluene. It belongs to the product categories of Pharmaceutical Intermediates; Aromatic Halides (substituted); Organics; Benzyl; Biochemistry; Reagents for Oligosaccharide Synthesis. Its EINECS registry number is 202-847-3. This chemical's molecular formula is C7H7Br and molecular weight is 171.03. What's more, both its IUPAC name and systematic name are the same which is called Bromomethylbenzene. It is an organic compound consisting of a benzene ring substituted with a bromomethyl group. This chemical is a colorless liquid that is decomposed slowly in water.
Physical properties about Benzyl bromide are: (1)ACD/LogP: 2.81; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.805; (4)ACD/LogD (pH 7.4): 2.805; (5)ACD/BCF (pH 5.5): 79.805; (6)ACD/BCF (pH 7.4): 79.805; (7)ACD/KOC (pH 5.5): 799.993; (8)ACD/KOC (pH 7.4): 799.993; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 0 Å2; (13)Index of Refraction: 1.568; (14)Molar Refractivity: 38.901 cm3; (15)Molar Volume: 118.926 cm3; (16)Surface Tension: 38.493 dyne/cm; (17)Density: 1.438 g/cm3; (18)Flash Point: 86.667 °C; (19)Enthalpy of Vaporization: 41.693 kJ/mol; (20)Boiling Point: 198.499 °C at 760 mmHg; (21)Vapour Pressure: 0.506 mmHg at 25 °C.
Preparation of Benzyl bromide: this chemical can be prepared by Benzyloxy-tert-butyl-dimethylsilan. This reaction needs reagent BBr3 and solvent CH2Cl2 at ambient temperature. The reaction time is 10 min. The yield is 90 %.

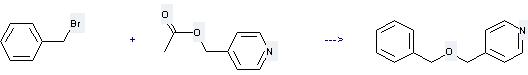
Uses of Benzyl bromide: (1) It is used in organic synthesis for the introduction of the benzyl protecting group for alcohols and carboxylic acids. It is also used as a war gas. (2) it is used to produce other chemicals. For example, it can react with 4-Acetoxymethyl-pyridine to get 4-Benzyloximethyl-pyridine. The reaction occurs with reagents 18-crown-6, KOH and solvent benzene at ambient temperature. The yield is 87 %.
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. Therefore, you should wear eye/face protection and keep out of the reach of children.
You can still convert the following datas into molecular structure:
(1) SMILES: c1ccc(cc1)CBr
(2) InChI: InChI=1S/C7H7Br/c8-6-7-4-2-1-3-5-7/h1-5H,6H2
(3) InChIKey: AGEZXYOZHKGVCM-UHFFFAOYSA-N
Related Products
- Benzyl (1-(aminocarbonyl)-2-hydroxypropyl)carbamate
- Benzyl (1-cyano-1-methylethyl)carbamate
- Benzyl (1R,2S)-3-chloro-2-hydroxy-1-(phenylthiomethyl)propylcarbamate
- Benzyl (2,5-dioxo-1,3-oxazolidin-4-yl)acetate
- Benzyl (2R,3S)-(-)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- Benzyl (2R,3S)-(1-carbamoyl-2-hydroxypropyl)carbamate
- Benzyl (2S,3aR,7aS)-octahydroindole-2-carboxylate hydrochloride
- Benzyl (2S,3aR,7aS)-octahydroindole-2-carboxylate hydrochloride
- Benzyl (2S,3R)-(+)-6-oxo-2,3-diphenyl-4-morpholinecarboxylate
- Benzyl (3-aminopropyl)carbamate
- 1003-91-4
- 10039-26-6
- 10039-32-4
- 10039-54-0
- 10039-56-2
- 1003-98-1
- 1003-99-2
- 1004-00-8
- 100402-39-9
- 100402-45-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View