-
Name
Betaxolol
- EINECS
- CAS No. 63659-18-7
- Article Data13
- CAS DataBase
- Density 1.067 g/cm3
- Solubility
- Melting Point 61-63 °C
- Formula C18H29NO3
- Boiling Point 448 °C at 760 mmHg
- Molecular Weight 307.433
- Flash Point 224.724 °C
- Transport Information
- Appearance White crystalline solid
- Safety
- Risk Codes R36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1-Isopropylamino-3-[4-(2-cyclopropylmethoxy-ethyl)phenoxy]-2-propanol;Betaxolol;Betoptic S;
- PSA 50.72000
- LogP 2.78430
Synthetic route
-
-
75-31-0
isopropylamine

-
-
63659-17-6
1-[2-(cyclopropyl-methoxy)-ethyl]-4-(2,3-epoxypropoxy)-benzene

-
-
63659-18-7
betaxolol

| Conditions | Yield |
|---|---|
| for 24h; Ambient temperature; | 90% |
| for 48h; Heating; Yield given; | |
| In water at 0 - 35℃; |
-
-
884340-61-8
RS 1-{4-[2-(allyloxy)-ethyl]phenoxy}-3-isopropylamino propan-2-ol

-
-
75-11-6
diiodomethane

-
-
63659-18-7
betaxolol

| Conditions | Yield |
|---|---|
| With diethylzinc In toluene at 0℃; for 16h; | 84% |

-
-
63659-18-7
betaxolol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In isopropyl alcohol | 74% |
-
-
63659-16-5
4-[2-(cyclopropylmethoxy)-ethyl]-phenol

-
-
63659-18-7
betaxolol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaOH / H2O / 12 h / Ambient temperature 2: 48 h / Heating View Scheme |
-
-
63659-15-4
1-benzyloxy-4-(2-cyclopropylmethoxyethyl)benzene

-
-
63659-18-7
betaxolol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 84 percent / H2 / 5percent Pd/C / tetrahydrofuran / 50 - 60 °C / 2585.7 Torr 2: NaOH / H2O / 12 h / Ambient temperature 3: 48 h / Heating View Scheme |
-
-
61439-59-6
2-(4-benzyloxyphenyl)ethanol

-
-
63659-18-7
betaxolol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.) NaH / 1.) DMF, 2.) DMF, 60 deg, 18 h 2: 84 percent / H2 / 5percent Pd/C / tetrahydrofuran / 50 - 60 °C / 2585.7 Torr 3: NaOH / H2O / 12 h / Ambient temperature 4: 48 h / Heating View Scheme |
-
-
56441-69-1
2-<4-(phenylmethoxy)phenyl>acetic acid ethyl ester

-
-
63659-18-7
betaxolol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 86 percent / LiAlH4 / tetrahydrofuran / 3 h / Ambient temperature 2: 1.) NaH / 1.) DMF, 2.) DMF, 60 deg, 18 h 3: 84 percent / H2 / 5percent Pd/C / tetrahydrofuran / 50 - 60 °C / 2585.7 Torr 4: NaOH / H2O / 12 h / Ambient temperature 5: 48 h / Heating View Scheme |
-
-
63659-19-8
betaxolol Hydrochloride

-
-
63659-18-7
betaxolol

| Conditions | Yield |
|---|---|
| With Dowex Marathon 11 chloride form In water |
-
-
63659-18-7
betaxolol

-
-
108-24-7
acetic anhydride

-
-
169613-96-1
1-(N-acetyl-N-isopropyl)amino-3-<4-(2-cyclopropylmethoxy)ethyl>phenoxyprop-2-yl acetate

| Conditions | Yield |
|---|---|
| With pyridine for 24h; Ambient temperature; | 100% |
| With pyridine; triethylamine at 20℃; |
-
-
63659-18-7
betaxolol

-
-
108-24-7
acetic anhydride

-
B
-
169613-97-2
Acetic acid (S)-2-(acetyl-isopropyl-amino)-1-[4-(2-cyclopropylmethoxy-ethyl)-phenoxymethyl]-ethyl ester

| Conditions | Yield |
|---|---|
| With pyridine for 24h; Ambient temperature; Yield given. Yields of byproduct given; |

-
-
63659-18-7
betaxolol

-
-
4023-34-1
cyclopropanecarboxylic acid chloride

| Conditions | Yield |
|---|---|
| Acylation; |
-
-
63659-18-7
betaxolol

-
-
63659-19-8
betaxolol Hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In acetone at 10 - 15℃; pH=2.0; |
-
-
63659-18-7
betaxolol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: pyridine; triethylamine / 20 °C 2: Rhodotorula mucilaginosa DQ832198 / 12 h / 28 °C / pH 6 / Microbiological reaction; phosphate-citric acid buffer 3: sodium hydroxide / methanol / 20 °C 4: hydrogenchloride / water / 20 °C View Scheme |
-
-
63659-18-7
betaxolol

-
-
93221-48-8
(-)-Betaxolol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: pyridine; triethylamine / 20 °C 2: Rhodotorula mucilaginosa DQ832198 / 12 h / 28 °C / pH 6 / Microbiological reaction; phosphate-citric acid buffer 3: sodium hydroxide / methanol / 20 °C View Scheme |
-
-
63659-18-7
betaxolol

-
-
169613-97-2
Acetic acid (S)-2-(acetyl-isopropyl-amino)-1-[4-(2-cyclopropylmethoxy-ethyl)-phenoxymethyl]-ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine; triethylamine / 20 °C 2: Rhodotorula mucilaginosa DQ832198 / 12 h / 28 °C / pH 6 / Microbiological reaction; phosphate-citric acid buffer View Scheme |

| Conditions | Yield |
|---|---|
| With diethylamine In acetonitrile Reagent/catalyst; Resolution of racemate; |
Betaxolol Chemical Properties
Molecular Formula: C18H29NO3
Molar mass: 307.43 g/mol
Density: 1.067 g/cm3
Flash Point: 224.7 °C
Index of Refraction: 1.529
Boiling Point: 448 °C at 760 mmHg
Vapour Pressure: 8.26E-09 mmHg at 25°C
Melting point: 61-63°C
Appearance: White crystalline solid
Product categories of Betaxolol (63659-18-7): Pharmaceuticals;Intermediates & Fine Chemicals;Steroids
Structure of Betaxolol (63659-18-7):
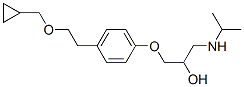
Systematic Name: 1-[4-[2-(Cyclopropylmethoxy)ethyl]phenoxy]-3-(isopropylamino)propan-2-ol
SMILES: O(CCc1ccc(OCC(O)CNC(C)C)cc1)CC2CC2
InChI: InChI=1/C18H29NO3/c1-14(2)19-11-17(20)13-22-18-7-5-15(6-8-18)9-10-21-12-16-3-4-16/h5-8,14,16-17,19-20H,3-4,9-13H2,1-2H3
InChIKey: NWIUTZDMDHAVTP-UHFFFAOYAU
Std. InChI: InChI=1S/C18H29NO3/c1-14(2)19-11-17(20)13-22-18-7-5-15(6-8-18)9-10-21-12-16-3-4-16/h5-8,14,16-17,19-20H,3-4,9-13H2,1-2H3
Std. InChIKey: NWIUTZDMDHAVTP-UHFFFAOYSA-N
Betaxolol History
Betaxolol (63659-18-7) was approved Drug Administration (FDA) by and the U.S. Food for ocular use as a 0.5% solution (Betoptic) in 1985 and as a 0.25% solution (Betoptic S) in 1989.
Betaxolol Uses
Betaxolol (63659-18-7) is a selective beta1 receptor blocker used in the treatment of hypertension and glaucoma. And it can be used for the management of hypertension and for the management of glaucoma .
Betaxolol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | oral | 60mg/kg (60mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 18(Suppl, |
Betaxolol Specification
Betaxolol (63659-18-7) also can be called Betoptic , Betoptic S , Lokren ,and Kerlone .It typically has fewer systemic side effects than non-selective beta-blockers, for example, not causing bronchospasm (mediated by beta2 receptors) as timolol may.In addition to its effect on the heart, betaxolol reduces the pressure within the eye (intraocular pressure). This effect is thought to be caused by reducing the production of the liquid (which is called the aqueous humor) within the eye. The precise mechanism of this effect is not known. The reduction in intraocular pressure reduces the risk of damage to the optic nerve and loss of vision in patients with elevated intraocular pressure due to glaucoma.It also shows greater affininty for beta1 receptors than metoprolol.
Related Products
- Betaxolol
- Betaxolol Hydrochloride
- Betaxolol hydrochloride
- 63659-19-8
- 636601-27-9
- 636601-30-4
- 636-61-3
- 636-65-7
- 63665-87-2
- 63666-10-4
- 63666-11-5
- 6366-70-7
- 636-70-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View