-
Name
Bicine
- EINECS 205-755-1
- CAS No. 150-25-4
- Article Data22
- CAS DataBase
- Density 1.31 g/cm3
- Solubility water: 1 M at 20 °C, clear, colorless
- Melting Point 190 °C (dec.)(lit.)
- Formula C6H13NO4
- Boiling Point 388.5 °C at 760 mmHg
- Molecular Weight 163.174
- Flash Point 188.8 °C
- Transport Information
- Appearance White crystalline powder
- Safety 24/25-36-26
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms N,N-Bis(2-hydroxyethyl)glycine;Bis(2-Hydroxyethyl)glycine;N,N-Bis(2-hydroxyethyl)aminoacetic acid;NSC 7342;Diethanol glycine;
- PSA 81.00000
- LogP -1.64240
Synthetic route

| Conditions | Yield |
|---|---|
| In ethanol; water at 70℃; for 16h; | 96% |

| Conditions | Yield |
|---|---|
| at 25℃; | |
| at 25℃; im Rohr; |

| Conditions | Yield |
|---|---|
| With water at 25℃; Rate constant; at pD 7.0; hydrolysis also in humid air; | |
| With water at 120℃; | |
| With water (heating); | |
| With water |
-
-
98486-41-0
N,N-bis-(2-chloro-ethyl)-glycine

-
A
-
86240-92-8
4-(2-chloro-ethyl)-morpholin-2-one

-
B
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; benzene | |
| With sodium hydrogencarbonate |

| Conditions | Yield |
|---|---|
| With methanol |
-
-
3926-62-3
sodium monochloroacetic acid

-
-
111-42-2
2,2'-iminobis[ethanol]

-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

| Conditions | Yield |
|---|---|
| With water |

| Conditions | Yield |
|---|---|
| With ethanol | |
| In ethanol |

| Conditions | Yield |
|---|---|
| With water at 25℃; Rate constant; Product distribution; Mechanism; pH varied: 4 to 8; hydrolysis of C-terminal amide group of various α-aminoacids having one or two hydroxyethyl groups at N-terminal, via cyclization to a morpholinolactone; reaction monitored by 1H NMR; |

-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

| Conditions | Yield |
|---|---|
| With potassium carbonate |

| Conditions | Yield |
|---|---|
| With water und Hydrolyse des Reaktionsprodukts mit wss.HCl; |

-
-
96-32-2
bromoacetic acid methyl ester

-
-
111-42-2
2,2'-iminobis[ethanol]

-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; triethylamine In propan-1-ol; ethanol |
-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
20427-59-2
copper hydroxide

-
-
77180-36-0, 921625-45-8, 13978-30-8
copper(II) bis(N,N-bis(2-hydroxyethyl)glycinate)

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) grinding at room temp. in air for 30 min; crystn. from EtOH:H2O (1:1) for 2 d at room temp., elem. anal.; | 99% |
-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
27668-52-6
octadecyldimethyl[3-(trimethoxysilyl)propyl]ammonium chloride

| Conditions | Yield |
|---|---|
| Stage #1: N,N-bis-(2-hydroxyethyl)glycine; octadecyldimethyl[3-(trimethoxysilyl)propyl]ammonium chloride In N,N-dimethyl-formamide at 145℃; Stage #2: With sodium methylate In N,N-dimethyl-formamide | 95% |
-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
95-54-5
1,2-diamino-benzene

-
-
91646-60-5
N-(benzimidazol-2-ylmethyl)iminodiethanol

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 48h; Heating; | 93% |
| With hydrogenchloride; water |
-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
85825-95-2
amine

| Conditions | Yield |
|---|---|
| 85% |
-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
108-24-7
acetic anhydride

-
-
86351-49-7
4-(2-acetoxy-ethyl)-morpholin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine for 1h; Heating; | 83% |
| With pyridine Erwaermen des Reaktionsprodukts in Methanol oder in H2O; |
-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
42428-34-2
Phenyl-triethoxygerman

-
-
75713-38-1
1-phenyl-2,8,9-trioxa-5-aza-1-germatricyclo[3.3.3.0(1,5)]undecane-3-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide; benzene byproducts: CH3CH2OH; boiled for 2 h with distillation; cooled; filtered off; washed (benzene and ether); dried (vac.); IR; elem.anal.; | 72% |
-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
5865-91-8
Methyl-triaethoxy-germanium

-
-
75713-37-0
1-methyl-2,8,9-trioxa-5-aza-1-germatricyclo[3.3.3.0(1,5)]undecane-3-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide; benzene byproducts: CH3CH2OH; boiled for 2 h with distillation; cooled; filtered off; washed (benzene and ether); dried (vac.); IR; elem.anal.; | 69% |
-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

| Conditions | Yield |
|---|---|
| With [(CH3)4N]OH In methanol; water boiling (pH .apprx. 8, 10 min); elem. anal.; | 68.8% |

-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
194363-15-0
([Mn(N,N-bis(2-hydroxyethyl)glycinate)Cl]2*2(H2O))n

| Conditions | Yield |
|---|---|
| With Me4NOH In methanol dropwise addn. of Me4NOH to mixt. of Mn-salt and ligand; crystn. on slow evapn. (few d); elem. anal.; | 68% |
-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
5865-92-9
Ethyl-triethoxygermanium

-
-
87511-09-9
1-ethyl-2,8,9-trioxa-5-aza-1-germatricyclo[3.3.3.0(1,5)]undecane-3-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide; benzene byproducts: CH3CH2OH; boiled for 2 h with distillation; cooled; filtered off; washed (benzene and ether); dried (vac.); IR; elem.anal.; | 60% |

| Conditions | Yield |
|---|---|
| With barium dihydroxide In water byproducts: BaSO4; org. compd. and Ba compd. dissolved in deionised water under N2 then heated and stirred for 30 min, VO compd. added and react. mixt. stirred for30 min; BaSO4 filtered off, filtrate concd. in vac. to oily residue, washed withEt2O, solid filtered, washed with Et2O and dried in vac.; elem. anal.; | 58% |


-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
60245-55-8, 1269638-47-2
[CdCl(N,N-bis(2-hydroxyethyl)glycine(-1H))]*H2O

| Conditions | Yield |
|---|---|
| With LiOH*H2O In methanol; water byproducts: LiCl, H2O; soln. of Cd salt in H2O added to stirred soln. of ligand and LiOH*H2O inMeOH; layered with acetone; crystd. for 5 d; filtered; crystals washed with cold MeOH and acetone; dried in air; elem. anal.; | 55% |

| Conditions | Yield |
|---|---|
| In methanol byproducts: H2O; soln. of Cd salt in MeOH added to stirred soln. of ligand and NaOH in MeOH; refluxed for 25 min; concd. slowly by evapn. over 4 d; filtered; crystals washed with cold MeOH and Et2O; dried in air; elem. anal.; | 53% |

-
-
83149-20-6
[dichlorobis(triphenylphosphane)(η2-N-benzoylhydrazido(3-)-N',O)rhenium(V)]

-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

| Conditions | Yield |
|---|---|
| In methanol N2, an excess of amine, refluxed for 8 h; concd. (vac.), diethyl ether added, left for 2 d at room temp., crysts. washed (methanol), dried (vac.); elem. anal.; | 48% |
-
-
7791-07-3
sodium perchlorate monohydrate

-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
7732-18-5
water

-
-
5144-89-8
1,10-phenanthroline hydrate

| Conditions | Yield |
|---|---|
| In ethanol; water byproducts: CH3COOH, CH3COONa; a soln. of bicine in water was added to a soln. of Pr-compound in H2O, stirred, an ethanolic soln. of NaClO4 and phenanthroline were added, allowed to stand for 20 days at room temp.; filtered, washed with cold ethanol and dried in air; elem. anal.; | 45% |

-
-
7791-07-3
sodium perchlorate monohydrate

-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
7732-18-5
water

-
-
15280-57-6
erbium(III) acetate tetrahydrate

-
-
5144-89-8
1,10-phenanthroline hydrate

| Conditions | Yield |
|---|---|
| In ethanol; water byproducts: CH3COOH, CH3COONa; a soln. of bicine in water was added to a soln. of Er-compound in H2O, stirred, an ethanolic soln. of NaClO4 and phenanthroline were added, allowed to stand for 20 days at 5°C; crystals were washed with cold ethanol, diethyl ether and dried in air; elem. anal.; | 40% |


| Conditions | Yield |
|---|---|
| In acetonitrile byproducts: PhCO2H, H2O; to a soln. of Fe3-contg. compd. (0.25 mmol) in MeCN was added with stirring a soln. of a ligand (0.50 mmol) in MeCN; the soln. was stirred overnight at ambient temp.; the ppt. was collected by filtration, washed with MeCN and dissolved in MeOH-CH2Cl2 (1:1); the soln. was layered with Et2O and left at ambient temp.; after 1 wk the crystals were collected by filtration, washed with CH2Cl2 and dried in vac.; elem. anal.; | 38% |

-
-
7791-07-3
sodium perchlorate monohydrate

-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
7732-18-5
water

-
-
14462-42-1, 16648-22-9, 17829-84-4, 25721-95-3, 25722-01-4, 50484-87-2, 61907-70-8, 92457-91-5
neodymium(III) acetate tetrahydrate

-
-
5144-89-8
1,10-phenanthroline hydrate

| Conditions | Yield |
|---|---|
| In ethanol; water byproducts: CH3COOH, CH3COONa; a soln. of bicine in water was added to a soln. of Nd-compound in H2O, stirred, an ethanolic soln. of NaClO4 and phenanthroline were added, allowed to stand for 20 days at room temp.; filtered, washed with cold ethanol and dried in air; elem. anal.; | 30% |

-
-
7791-07-3
sodium perchlorate monohydrate

-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
7732-18-5
water

-
-
14432-01-0, 15280-53-2, 79464-08-7, 100587-93-7, 138481-01-3
gadolinium(III) acetate tetrahydrate

-
-
5144-89-8
1,10-phenanthroline hydrate

| Conditions | Yield |
|---|---|
| In ethanol; water byproducts: CH3COOH, CH3COONa; a soln. of bicine in water was added to a soln. of Gd-compound in H2O, stirred, an ethanolic soln. of NaClO4 and phenanthroline were added, allowed to stand for 20 days at room temp.; filtered, washed with cold ethanol and dried in air; elem. anal.; | 25% |


-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 140℃; under 15514.9 Torr; for 0.25h; Sealed tube; Microwave irradiation; | 18% |

-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 140℃; under 15514.9 Torr; for 0.25h; Sealed tube; Microwave irradiation; | 17% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 140℃; under 15514.9 Torr; for 0.25h; Sealed tube; Microwave irradiation; | 17% |


| Conditions | Yield |
|---|---|
| In acetonitrile N,N-bis(2-hydroxyethyl)glycine added to MeCN soln. of Fe pivalate deriv.followed by Et2NH (1:1:1); stirred at ambient temp. for 24 h; filtered; cryst. in a sealed vial after 2 wks; elem. anal.; | 16% |


| Conditions | Yield |
|---|---|
| With NaOMe In acetonitrile N,N-bis(2-hydroxyethyl)glycine added to MeCN soln. of Fe pivalate deriv.followed by NaOMe (1:1:1); stirred at ambient temp. for 24 h; filtered; cryst. in a sealed vial after 2 wks; elem. anal.; | 12% |

-
-
5348-42-5
4,5-Dichloro-1,2-phenylenediamine

-
-
150-25-4
N,N-bis-(2-hydroxyethyl)glycine

-
-
6478-89-3
2,2'-(5,6-dichloro-1H-benzoimidazol-2-ylmethylazanediyl)-bis-ethanol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water |

| Conditions | Yield |
|---|---|
| durch trockne Destillation; | |
| With hydrogenchloride | |
| With hydrogenchloride | |
| In solid at 195℃; for 0.166667h; |
Bicine Specification
The Bicine is an organic compound with the formula C6H13NO4. The IUPAC name of this chemical is 2-[bis(2-hydroxyethyl)amino]acetic acid. With the CAS registry number 150-25-4 and EINECS 205-755-1, it is also named as N,N-Bis(2-hydroxyethyl)glycine. The product's categories are Biochemistry; Good's Buffers; Buffer. It is white crystalline powder which is stable under normal temperature and pressure. Additionally, this chemical should be stored at normal temperature.
The other characteristics of this product can be summarized as: (1)ACD/LogP: -0.48; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -2.96; (4)ACD/LogD (pH 7.4): -2.99; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 5; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 8; (12)Polar Surface Area: 48 Å2; (13)Index of Refraction: 1.525; (14)Molar Refractivity: 38.2 cm3; (15)Molar Volume: 124.4 cm3; (16)Polarizability: 15.14×10-24 cm3; (17)Surface Tension: 63.8 dyne/cm; (18)Density: 1.31 g/cm3; (19)Flash Point: 188.8 °C; (20)Enthalpy of Vaporization: 73.75 kJ/mol; (21)Boiling Point: 388.5 °C at 760 mmHg; (22)Vapour Pressure: 1.21E-07 mmHg at 25°C.
Preparation of Bicine: It can be obtained by 2,2'-azanediyl-bis-ethanol and glyoxal. This reaction needs solvents H2O, ethanol at temperature of 70 °C. The reaction time is 16 hours. The yield is 96%.
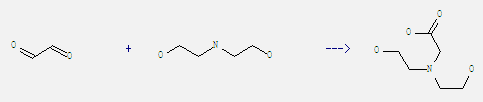
Uses of Bicine: It is used as a buffering agent in biochemical research. And it can react with acetic acid anhydride to get 4-(2-acetoxy-ethyl)-morpholin-2-one. This reaction needs reagent Et3N by heating. The reaction time is 1 hours. The yield is 83%.

When you are using this chemical, please be cautious about it as the following:
It is not only harmful by inhalation, in contact with skin and if swallowed, but also irritating to eyes, respiratory system and skin. So people should avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If you want to contact this product, you must wear suitable protective clothing.
People can use the following data to convert to the molecule structure.
1. SMILES:O=C(O)CN(CCO)CCO
2. InChI:InChI=1/C6H13NO4/c8-3-1-7(2-4-9)5-6(10)11/h8-9H,1-5H2,(H,10,11)
3. InChIKey:FSVCELGFZIQNCK-UHFFFAOYAV
The following are the toxicity data which has been tested.
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 1540mg/kg (1540mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | Revue d'Epidemiologie, Medecine Sociale et Sante Publique. Vol. 10, Pg. 391, 1962. |
Related Products
- Bicine
- 150255-96-2
- 150256-42-1
- 150258-63-2
- 15026-17-2
- 15027-14-2
- 15028-10-1
- 150281-45-1
- 15028-39-4
- 15028-41-8
- 150285-07-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View