-
Name
Bifonazole
- EINECS 262-336-6
- CAS No. 60628-96-8
- Article Data15
- CAS DataBase
- Density 1.077 g/cm3
- Solubility
- Melting Point 142℃
- Formula C22H18N2
- Boiling Point 491.728 °C at 760 mmHg
- Molecular Weight 310.398
- Flash Point 251.188 °C
- Transport Information
- Appearance
- Safety
- Risk Codes 22
-
Molecular Structure
- Hazard Symbols Xn
- Synonyms 1-[4-Biphenylyl(phenyl)methyl]-1H-imidazole;
- PSA 17.82000
- LogP 5.18780
Synthetic route
-
-
162824-42-2
(R,S)-1-<α-(4-Biphenylyl)benzyl>imidazole-4,5-dicarboxylic acid

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| In diphenylether for 0.5h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-benzylbiphenyl With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In tetrachloromethane for 0.833333h; Inert atmosphere; Reflux; Stage #2: 1H-imidazole With potassium carbonate In acetonitrile for 1h; Reflux; | 77% |
| Stage #1: 4-benzylbiphenyl With pyridine; tert.-butylhydroperoxide; iodine In decane; water at 80℃; for 12h; Schlenk technique; Inert atmosphere; Stage #2: 1H-imidazole With formic acid; toluene-4-sulfonic acid In water at 180 - 200℃; for 10h; Schlenk technique; Inert atmosphere; | 66% |

| Conditions | Yield |
|---|---|
| With copper acetylacetonate; tetrabutylammomium bromide In toluene at 85℃; for 36h; | 52% |

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one Heating; | 12% |

| Conditions | Yield |
|---|---|
| With bis(dicyclohexylphenylphosphine)nickel(II) chloride In diethyl ether at 80℃; for 15h; |
-
-
7598-80-3
4-phenylbenzhydrol

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 96 percent / DEAD, Ph3P / tetrahydrofuran / 24 h / Ambient temperature 2: 85 percent / NaOH / ethanol / 24 h / Heating 3: 98 percent / diphenyl ether / 0.5 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1.1: triethylamine / dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere 2.1: sodium hydride / N,N-dimethyl-formamide; mineral oil / 2 h / 0 °C / Inert atmosphere 2.2: 0 - 100 °C / Inert atmosphere View Scheme |
-
-
162824-36-4
1-<α-(4-Biphenylyl)benzyl>imidazole-4,5-dicarbonitrile

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / NaOH / ethanol / 24 h / Heating 2: 98 percent / diphenyl ether / 0.5 h / Heating View Scheme |
-
-
7515-73-3
4-(chlorophenylmethyl)-1,1'-biphenyl

-
-
18156-74-6
1-(Trimethylsilyl)imidazole

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| In acetonitrile |

| Conditions | Yield |
|---|---|
| Stage #1: 1H-imidazole; 4-phenylbenzhydrol With ammonium bromide In water at 125 - 130℃; for 2h; Industrial scale; Stage #2: With ammonium bromide In water at 190 - 195℃; for 2h; Temperature; Industrial scale; |
-
-
768-32-1
trimethylphenylsilane

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: silver(I) 4-methylbenzenesulfonate; dichloro(N-(diphenylphosphino)-N-isopropyl-1,1-diphenylphosphinamine) digold(I); [bis(acetoxy)iodo]benzene / 1,1,1-trichloroethane / 2 h / 110 °C / Inert atmosphere; Schlenk technique 2.1: aluminum oxide; sodium tetrahydroborate / ethanol / 0.67 h / 20 °C / Inert atmosphere 3.1: triethylamine / dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere 4.1: sodium hydride / N,N-dimethyl-formamide; mineral oil / 2 h / 0 °C / Inert atmosphere 4.2: 0 - 100 °C / Inert atmosphere View Scheme |
-
-
1063965-82-1
(4-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)phenyl)(phenyl)methanone

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: silver(I) 4-methylbenzenesulfonate; dichloro(N-(diphenylphosphino)-N-isopropyl-1,1-diphenylphosphinamine) digold(I); [bis(acetoxy)iodo]benzene / 1,1,1-trichloroethane / 2 h / 110 °C / Inert atmosphere; Schlenk technique 2.1: aluminum oxide; sodium tetrahydroborate / ethanol / 0.67 h / 20 °C / Inert atmosphere 3.1: triethylamine / dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere 4.1: sodium hydride / N,N-dimethyl-formamide; mineral oil / 2 h / 0 °C / Inert atmosphere 4.2: 0 - 100 °C / Inert atmosphere View Scheme |
-
-
2128-93-0
biphenyl-4-yl-phenyl-methanone

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: aluminum oxide; sodium tetrahydroborate / ethanol / 0.67 h / 20 °C / Inert atmosphere 2.1: triethylamine / dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere 3.1: sodium hydride / N,N-dimethyl-formamide; mineral oil / 2 h / 0 °C / Inert atmosphere 3.2: 0 - 100 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: 1H-imidazole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 2h; Inert atmosphere; Stage #2: [1,1'-biphenyl]-4-yl(phenyl)methyl methanesulfonate In N,N-dimethyl-formamide; mineral oil at 0 - 100℃; Inert atmosphere; | 61.9 mg |
-
-
101-81-5
Diphenylmethane

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: dichloromethane / -40 - 20 °C / Schlenk technique; Inert atmosphere 2: bis(tri-t-butylphosphine)palladium(0); sodium hydrogencarbonate / dichloromethane; N,N-dimethyl-formamide / 12 h / 50 °C / Inert atmosphere; Schlenk technique 3: 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / tetrachloromethane / 0.83 h / Inert atmosphere; Reflux 4: potassium carbonate / acetonitrile / 1 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: dichloromethane / -40 - 20 °C / Inert atmosphere; Schlenk technique 2.1: sodium hydrogencarbonate; bis(tri-t-butylphosphine)palladium(0) / dichloromethane; N,N-dimethyl-formamide / 12 h / 50 °C / Inert atmosphere; Schlenk technique; Sealed tube 3.1: N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) / tetrachloromethane / 0.83 h / Inert atmosphere; Reflux 3.2: 1 h / Reflux View Scheme |

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: bis(tri-t-butylphosphine)palladium(0); sodium hydrogencarbonate / dichloromethane; N,N-dimethyl-formamide / 12 h / 50 °C / Inert atmosphere; Schlenk technique 2: 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / tetrachloromethane / 0.83 h / Inert atmosphere; Reflux 3: potassium carbonate / acetonitrile / 1 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 1h; Reflux; | 47.9 mg |
-
-
613-42-3
4-benzylbiphenyl

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / tetrachloromethane / 0.83 h / Inert atmosphere; Reflux 2: potassium carbonate / acetonitrile / 1 h / Reflux View Scheme |
-
-
24388-23-6
2-phenyl-4,4,5,5-tetramethyl-1,3,2-dioxoborole

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydrogencarbonate; bis(tri-t-butylphosphine)palladium(0) / dichloromethane; N,N-dimethyl-formamide / 12 h / 50 °C / Inert atmosphere; Schlenk technique; Sealed tube 2.1: N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) / tetrachloromethane / 0.83 h / Inert atmosphere; Reflux 2.2: 1 h / Reflux View Scheme |

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: tetrahydrofuran / 0.08 h / 20 °C / Schlenk technique; Inert atmosphere 1.2: 12.03 h / 70 °C / Schlenk technique; Inert atmosphere; Glovebox 2.1: iodine; pyridine; tert.-butylhydroperoxide / water; decane / 12 h / 80 °C / Schlenk technique; Inert atmosphere 2.2: 10 h / 180 - 200 °C / Schlenk technique; Inert atmosphere View Scheme |
-
-
1135-12-2
p-benzylaniline

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine / dichloromethane / 12.5 h / -78 - 20 °C / Schlenk technique; Inert atmosphere 2.1: tetrahydrofuran / 0.08 h / 20 °C / Schlenk technique; Inert atmosphere 2.2: 12.03 h / 70 °C / Schlenk technique; Inert atmosphere; Glovebox 3.1: iodine; pyridine; tert.-butylhydroperoxide / water; decane / 12 h / 80 °C / Schlenk technique; Inert atmosphere 3.2: 10 h / 180 - 200 °C / Schlenk technique; Inert atmosphere View Scheme |
-
-
67969-82-8
tetrafluoroboric acid diethyl ether

-
-
13755-29-8
sodium tetrafluoroborate

-
-
2362-50-7
thianthrene-5-oxide

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| Stage #1: tetrafluoroboric acid diethyl ether; thianthrene-5-oxide; bifonazole With trifluoroacetic anhydride In acetonitrile at 0 - 25℃; for 18h; Schlenk technique; Stage #2: sodium tetrafluoroborate In dichloromethane; water | 95% |

-
-
5798-75-4
Ethyl 4-bromobenzoate

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 90% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; 2,3,7,8-tetrafluorothianthrene; trifluoroacetic anhydride In acetonitrile at 0 - 25℃; regioselective reaction; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: trifluorormethanesulfonic acid; 2,3,7,8-tetrafluorothianthrene-S-oxide; bifonazole With trifluoroacetic anhydride In acetonitrile at 0 - 25℃; for 3h; Sealed tube; Stage #2: sodium triflate In ethanol; dichloromethane; water | 87% |

-
-
10342-83-3
4'-Bromopropiophenone

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 77% |
-
-
1122-91-4
4-bromo-benzaldehyde

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 77% |
-
-
401-78-5
3-bromo-1-trifluoromethylbenzene

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 71% |
-
-
402-43-7
p-trifluoromethylphenyl bromide

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 66% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic anhydride In acetonitrile at -40 - 25℃; for 2h; Schlenk technique; Glovebox; Inert atmosphere; | 65% |

-
-
1532-97-4
4-bromoisoquinoline

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 63% |
-
-
623-00-7
4-bromobenzenecarbonitrile

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 61% |
-
-
6952-59-6
3-cyanobromobenzene

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 61% |
-
-
5370-25-2
2-Acetyl-5-bromothiophene

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 59% |
-
-
2042-37-7
o-cyanobromobenzene

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 58% |
-
-
106-38-7
para-bromotoluene

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With cesium acetate; palladium diacetate at 150℃; for 48h; Inert atmosphere; Schlenk technique; | A 58% B n/a |

-
-
104-92-7
1-bromo-4-methoxy-benzene

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With cesium acetate; palladium diacetate at 150℃; for 48h; Inert atmosphere; Schlenk technique; | A 57% B n/a |

-
-
108-86-1
bromobenzene

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With cesium acetate; palladium diacetate at 150℃; for 48h; Inert atmosphere; Schlenk technique; | A 55% B n/a |


-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With air In chloroform treatment of Co-complex with rac-bifonazole; XRD, UV-Vis; | 46% |
-
-
392-83-6
o-trifluoromethylphenyl bromide

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 45% |
-
-
626-60-8
3-Chloropyridine

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 45% |
-
-
60628-96-8
bifonazole

-
-
586-78-7
para-nitrophenyl bromide

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 43% |
-
-
90-11-9
1-Bromonaphthalene

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 42% |
-
-
4595-59-9
5-bromopyrimidine

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 41% |
-
-
580-13-2
2-bromonaphthalene

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 39% |
-
-
3972-65-4
1-bromo-4-tert-butylbenzene

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 34% |
-
-
2635-13-4
1,2-(methylenedioxy)-4-bromobenzene

-
-
60628-96-8
bifonazole

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate at 150℃; for 16h; Inert atmosphere; Schlenk technique; | 28% |
Bifonazole Specification
The Bifonazole, with the CAS registry number 60628-96-8, is also known as 1-[4-Biphenylyl(phenyl)methyl]-1H-imidazole. It belongs to the classification codes of Anti-Infective Agents; Antifungal; Antifungal Agents; Drug / Therapeutic Agent; Reproductive Effect. Its EINECS registry number is 262-336-6. This chemical's molecular formula is C22H18N2 and molecular weight is 310.39. What's more, its IUPAC name is called 1-[Phenyl-(4-phenylphenyl)methyl]imidazole. It should be stored in a cool, dry and well-ventilated place. Bifonazole is topical imidazole antifungal drug for the treatment of a variety of dermatophytoses, such as tinea corporis, tinea cruris, hand, foot and ringworm.
Physical properties about Bifonazole are: (1)ACD/LogP: 4.689; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.62; (4)ACD/LogD (pH 7.4): 4.63; (5)ACD/BCF (pH 5.5): 182.76; (6)ACD/BCF (pH 7.4): 1890.91; (7)ACD/KOC (pH 5.5): 717.78; (8)ACD/KOC (pH 7.4): 7426.47; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 17.82 Å2; (13)Index of Refraction: 1.616; (14)Molar Refractivity: 100.752 cm3; (15)Molar Volume: 288.133 cm3; (16)Polarizability: 39.941×10-24cm3; (17)Surface Tension: 43.15 dyne/cm; (18)Density: 1.077 g/cm3; (19)Flash Point: 251.188 °C; (20)Enthalpy of Vaporization: 72.969 kJ/mol; (21)Boiling Point: 491.728 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25 °C.
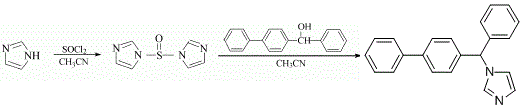
Preparation of Bifonazole: this chemical can be prepared by imidazole with thionyl chloride. This reaction needs reagent acetonitrile at ambient temperature. The reaction time is 15 hours.
You can still convert the following datas into molecular structure:
(1) SMILES: n1ccn(c1)C(c3ccc(c2ccccc2)cc3)c4ccccc4
(2) InChI: InChI=1S/C22H18N2/c1-3-7-18(8-4-1)19-11-13-21(14-12-19)22(24-16-15-23-17-24)20-9-5-2-6-10-20/h1-17,22H
(3) InChIKey: OCAPBUJLXMYKEJ-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | oral | > 500mg/kg (500mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 33, Pg. 739, 1983. | |
| mouse | LD50 | intravenous | 57mg/kg (57mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Oyo Yakuri. Pharmacometrics. Vol. 28, Pg. 23, 1984. |
| mouse | LD50 | oral | 2629mg/kg (2629mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 33, Pg. 739, 1983. | |
| mouse | LD50 | subcutaneous | > 15gm/kg (15000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Oyo Yakuri. Pharmacometrics. Vol. 28, Pg. 23, 1984. |
| rabbit | LD50 | oral | 4gm/kg (4000mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 33, Pg. 739, 1983. | |
| rat | LD50 | intravenous | 63mg/kg (63mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Oyo Yakuri. Pharmacometrics. Vol. 28, Pg. 23, 1984. |
| rat | LD50 | oral | 1463mg/kg (1463mg/kg) | SKIN AND APPENDAGES (SKIN): HAIR: OTHER BEHAVIORAL: ATAXIA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Oyo Yakuri. Pharmacometrics. Vol. 28, Pg. 23, 1984. |
| rat | LD50 | subcutaneous | > 10gm/kg (10000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Oyo Yakuri. Pharmacometrics. Vol. 28, Pg. 23, 1984. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View