-
Name
Boc-L-Hydroxyproline
- EINECS 604-011-7
- CAS No. 13726-69-7
- Article Data100
- CAS DataBase
- Density 1.312 g/cm3
- Solubility very faint turbidity
- Melting Point 123-127 °C(lit.)
- Formula C10H17NO5
- Boiling Point 390.9 °C at 760 mmHg
- Molecular Weight 231.249
- Flash Point 190.2 °C
- Transport Information
- Appearance
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Boc-trans-4-hydroxy-L-proline;4-Hydroxy-Pyrrolidine-1,2-Dicarboxylic Acid 1-tert-Butyl Ester;Boc-L-4-hydroxyproline;N-Boc-L-hydroxyproline;(2S,4R)-N-alpha-t-butoxycarbonyl-4-hydroxypyrrolidine-2-car boxylic acid;Boc-4-hydroxy-L-proline;Boc-L-Hyp-OH;Boc-(2S,4R)-hydroxyproline;
- PSA 87.07000
- LogP 0.37920
Synthetic route
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
51-35-4
4R-4-hydroxyproline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; acetone at 50℃; for 1h; | 100% |
| In tetrahydrofuran; water at 20℃; for 12h; | 100% |
| With sodium hydroxide In tetrahydrofuran; water at 20℃; | 100% |

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
51-35-4, 913000-57-4
4-hydroxyproline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; ethyl acetate | 96% |

-
-
74844-91-0
1-tert-butyl 2-methyl (2S,4R)-4-hydroxy-1,2-pyrrolidinedicarboxylate

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With water; lithium hydroxide In methanol at 20℃; for 1h; | 58% |
| With sodium hydroxide In tetrahydrofuran at 0℃; for 19h; | |
| Stage #1: 1-tert-butyl 2-methyl (2S,4R)-4-hydroxy-1,2-pyrrolidinedicarboxylate With water; sodium hydroxide In methanol for 4h; Stage #2: With citric acid In methanol; water pH=3; Product distribution / selectivity; | |
| Stage #1: 1-tert-butyl 2-methyl (2S,4R)-4-hydroxy-1,2-pyrrolidinedicarboxylate With methanol; water; lithium hydroxide at 20℃; Inert atmosphere; Stage #2: With hydrogenchloride In water; acetonitrile for 0.166667h; Cooling with ice bath; Inert atmosphere; |
-
-
34619-03-9
tert-butyldicarbonate

-
-
51-35-4
4R-4-hydroxyproline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane; water at 20℃; | 97% |
| With sodium hydroxide In water; tert-butyl alcohol at 20℃; for 16h; | 89% |
| With sodium hydrogencarbonate In water |
-
-
51-35-4
4R-4-hydroxyproline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| In water | 95% |
-
-
184046-78-4
(2S,3S)-1-tert-butyl-2-methyl 3-hydroxypyrrolidine-1,2-dicarboxylate

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (2S,3S)-1-tert-butyl-2-methyl 3-hydroxypyrrolidine-1,2-dicarboxylate With water; sodium hydroxide In methanol for 6h; Stage #2: With citric acid In methanol; water pH=3; |

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
51-35-4, 913000-57-4
4-hydroxyproline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| In sodium hydroxide; hexane; water; tert-butyl alcohol | |
| In sodium hydroxide; hexane; water; tert-butyl alcohol | |
| In sodium hydroxide; hexane; water; tert-butyl alcohol | |
| In sodium hydroxide; hexane; water; tert-butyl alcohol |

-
-
7732-18-5
water

-
-
51-35-4, 913000-57-4
4-hydroxyproline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane |
-
-
3236-56-4
dipercarbonate de O,O-t-butyle

-
-
51-35-4
4R-4-hydroxyproline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; ammonium chloride In tetrahydrofuran; water |
-
-
115352-39-1, 124662-61-9, 110339-26-9
(2R)-1-benzyloxy-2-hydroxy-pent-4-yne

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: hydrogen / Pd/CaCO3 / ethyl acetate 2: di-isopropyl azodicarboxylate, Ph3P / tetrahydrofuran / 12 h / -20 °C 3: hydrazine / ethanol / 6 h / Heating 4: Et3N / CH2Cl2 5: 78 percent / I2 / tetrahydrofuran; H2O / 6 h / 20 °C 6: Et3N / CH2Cl2 7: RuCl3*H2O, NaIO4, / CCl4; acetonitrile; H2O / 1 h / Ambient temperature 8: K2CO3 / methanol View Scheme |
-
-
84348-37-8
N-tert-butoxycarbonyl-4-oxo-L-proline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 90 percent / sodium borohydride / methanol / 3.5 h / 0 °C 2: 62 percent / aq. NaOH / diethyl ether / 24 h 3: 36 percent / lithium triethylborodeuteride / tetrahydrofuran / 24 h / Heating 4: trifluoroacetic acid / 0.5 h 5: TES buffer, iron(II)ammonium sulphate, 2-oxoglutarate, proline 4-hydroxylase / 4 h / 26 °C / incubation 6: 85 percent / aq. NaOH / 2-methyl-propan-2-ol / 18 h View Scheme |
-
-
87691-27-8
N-tert-butoxycarbonyl-L-cis-4-hydroxyproline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 62 percent / aq. NaOH / diethyl ether / 24 h 2: 36 percent / lithium triethylborodeuteride / tetrahydrofuran / 24 h / Heating 3: trifluoroacetic acid / 0.5 h 4: TES buffer, iron(II)ammonium sulphate, 2-oxoglutarate, proline 4-hydroxylase / 4 h / 26 °C / incubation 5: 85 percent / aq. NaOH / 2-methyl-propan-2-ol / 18 h View Scheme |
-
-
153790-69-3
(2S,4R)-<4-2H1>-proline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: TES buffer, iron(II)ammonium sulphate, 2-oxoglutarate, proline 4-hydroxylase / 4 h / 26 °C / incubation 2: 85 percent / aq. NaOH / 2-methyl-propan-2-ol / 18 h View Scheme |

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: trifluoroacetic acid / 0.5 h 2: TES buffer, iron(II)ammonium sulphate, 2-oxoglutarate, proline 4-hydroxylase / 4 h / 26 °C / incubation 3: 85 percent / aq. NaOH / 2-methyl-propan-2-ol / 18 h View Scheme |
-
-
153790-67-1
N-Boc-D-cis-4-(p-toluenesulfonyloxy)proline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 36 percent / lithium triethylborodeuteride / tetrahydrofuran / 24 h / Heating 2: trifluoroacetic acid / 0.5 h 3: TES buffer, iron(II)ammonium sulphate, 2-oxoglutarate, proline 4-hydroxylase / 4 h / 26 °C / incubation 4: 85 percent / aq. NaOH / 2-methyl-propan-2-ol / 18 h View Scheme |
-
-
90600-20-7
(2S)-2-[(tert-butoxycarbonyl)amino]pent-4-enoic acid

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 73 percent / N-bromosuccinimide / tetrahydrofuran / 0.33 h / 0 °C 2: 100 percent / K2CO3 / 0.5 h / 0 °C 3: 1) 0.5 N NaOH, 2) dl-10-camphorsulfonic acid / 1) THF, 0 deg C, 14 h, 2) CH2Cl2, RT, 20 h 4: 1) (COCl)2, DMSO, 2) NEt3 / 1a) CH2Cl2, -78 deg C, 15 min, 1b) -45 deg C, 1 h, 2a) -45 deg C, 20 min, 2b) 0 deg C, 15 min 5: 78 percent / p-TsOH*H2O / 14 h / Ambient temperature 6: 1) 60percent HOAc, 2) NaBH3CN, HOAc / 1) RT, 3 d, 2) EtOH, RT, 1 h 7: 0.5 N NaOH / tetrahydrofuran / 19 h / 0 °C View Scheme |
-
-
104241-30-7
(2S,4S)-2-(tert-butoxycarbonyl)amino-4-bromomethyl-γ-butyrolactone

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 100 percent / K2CO3 / 0.5 h / 0 °C 2: 1) 0.5 N NaOH, 2) dl-10-camphorsulfonic acid / 1) THF, 0 deg C, 14 h, 2) CH2Cl2, RT, 20 h 3: 1) (COCl)2, DMSO, 2) NEt3 / 1a) CH2Cl2, -78 deg C, 15 min, 1b) -45 deg C, 1 h, 2a) -45 deg C, 20 min, 2b) 0 deg C, 15 min 4: 78 percent / p-TsOH*H2O / 14 h / Ambient temperature 5: 1) 60percent HOAc, 2) NaBH3CN, HOAc / 1) RT, 3 d, 2) EtOH, RT, 1 h 6: 0.5 N NaOH / tetrahydrofuran / 19 h / 0 °C View Scheme |
-
-
104241-32-9
(2S,4R)-2-tert-butoxycarbonylamino-4-hydroxymethyl-4-butanolide

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1) (COCl)2, DMSO, 2) NEt3 / 1a) CH2Cl2, -78 deg C, 15 min, 1b) -45 deg C, 1 h, 2a) -45 deg C, 20 min, 2b) 0 deg C, 15 min 2: 78 percent / p-TsOH*H2O / 14 h / Ambient temperature 3: 1) 60percent HOAc, 2) NaBH3CN, HOAc / 1) RT, 3 d, 2) EtOH, RT, 1 h 4: 0.5 N NaOH / tetrahydrofuran / 19 h / 0 °C View Scheme |
-
-
106391-74-6
(2S,4R)-2-(tert-butoxycarbonyl)amino-4-formyl-γ-butyrolactone

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 78 percent / p-TsOH*H2O / 14 h / Ambient temperature 2: 1) 60percent HOAc, 2) NaBH3CN, HOAc / 1) RT, 3 d, 2) EtOH, RT, 1 h 3: 0.5 N NaOH / tetrahydrofuran / 19 h / 0 °C View Scheme |
-
-
104241-31-8
(2S,4S)-2-(tert-butoxycarbonyl)amino-3,4-epoxypentanoic acid methyl ester

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 1) 0.5 N NaOH, 2) dl-10-camphorsulfonic acid / 1) THF, 0 deg C, 14 h, 2) CH2Cl2, RT, 20 h 2: 1) (COCl)2, DMSO, 2) NEt3 / 1a) CH2Cl2, -78 deg C, 15 min, 1b) -45 deg C, 1 h, 2a) -45 deg C, 20 min, 2b) 0 deg C, 15 min 3: 78 percent / p-TsOH*H2O / 14 h / Ambient temperature 4: 1) 60percent HOAc, 2) NaBH3CN, HOAc / 1) RT, 3 d, 2) EtOH, RT, 1 h 5: 0.5 N NaOH / tetrahydrofuran / 19 h / 0 °C View Scheme |

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1) 60percent HOAc, 2) NaBH3CN, HOAc / 1) RT, 3 d, 2) EtOH, RT, 1 h 2: 0.5 N NaOH / tetrahydrofuran / 19 h / 0 °C View Scheme |
-
-
121795-00-4
(2S,4R)-4-(benzyloxy)-1-(tert-butoxycarbonyl)pyrrolidine-2-carboxylic acid

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol |
-
-
58931-16-1, 88981-35-5, 110339-28-1
(R)-1-benzyloxypent-4-en-2-ol

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: di-isopropyl azodicarboxylate, Ph3P / tetrahydrofuran / 12 h / -20 °C 2: hydrazine / ethanol / 6 h / Heating 3: Et3N / CH2Cl2 4: 78 percent / I2 / tetrahydrofuran; H2O / 6 h / 20 °C 5: Et3N / CH2Cl2 6: RuCl3*H2O, NaIO4, / CCl4; acetonitrile; H2O / 1 h / Ambient temperature 7: K2CO3 / methanol View Scheme |
-
-
121794-95-4
(S)-1-(benzyloxy)pent-4-en-2-amine

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: Et3N / CH2Cl2 2: 78 percent / I2 / tetrahydrofuran; H2O / 6 h / 20 °C 3: Et3N / CH2Cl2 4: RuCl3*H2O, NaIO4, / CCl4; acetonitrile; H2O / 1 h / Ambient temperature 5: K2CO3 / methanol View Scheme |
-
-
121794-96-5
N-((S)-1-Benzyloxymethyl-but-3-enyl)-benzamide

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 78 percent / I2 / tetrahydrofuran; H2O / 6 h / 20 °C 2: Et3N / CH2Cl2 3: RuCl3*H2O, NaIO4, / CCl4; acetonitrile; H2O / 1 h / Ambient temperature 4: K2CO3 / methanol View Scheme |
-
-
121794-97-6
O-benzyl-(2S,4R)-4-benzoyloxyprolinol

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Et3N / CH2Cl2 2: RuCl3*H2O, NaIO4, / CCl4; acetonitrile; H2O / 1 h / Ambient temperature 3: K2CO3 / methanol View Scheme |
-
-
121794-94-3
2-((S)-1-Benzyloxymethyl-but-3-enyl)-isoindole-1,3-dione

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: hydrazine / ethanol / 6 h / Heating 2: Et3N / CH2Cl2 3: 78 percent / I2 / tetrahydrofuran; H2O / 6 h / 20 °C 4: Et3N / CH2Cl2 5: RuCl3*H2O, NaIO4, / CCl4; acetonitrile; H2O / 1 h / Ambient temperature 6: K2CO3 / methanol View Scheme |
-
-
121794-98-7
(2S,4R)-4-Benzoyloxy-2-benzyloxymethyl-pyrrolidine-1-carboxylic acid tert-butyl ester

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: RuCl3*H2O, NaIO4, / CCl4; acetonitrile; H2O / 1 h / Ambient temperature 2: K2CO3 / methanol View Scheme |
-
-
51-35-4
4R-4-hydroxyproline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With triethylamine |
-
-
1070-19-5
N-(tert-butyloxycarbonyl) azide

-
-
51-35-4
4R-4-hydroxyproline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
74844-91-0
1-tert-butyl 2-methyl (2S,4R)-4-hydroxy-1,2-pyrrolidinedicarboxylate

| Conditions | Yield |
|---|---|
| In methanol | 100% |
| In methanol; diethyl ether | 100% |
| In tetrahydrofuran | 96% |
-
-
100-39-0
benzyl bromide

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
40350-83-2, 54631-82-2, 54631-81-1
(2S,4R)-4-(benzyloxy)-1-(tert-butoxycarbonyl)pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With sodium hydride In tetrahydrofuran; dimethyl sulfoxide; mineral oil at -20 - 0℃; Inert atmosphere; Stage #2: benzyl bromide In tetrahydrofuran; dimethyl sulfoxide; mineral oil at -20 - 25℃; for 6h; Inert atmosphere; chemoselective reaction; | 100% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 1h; Inert atmosphere; Stage #2: benzyl bromide In tetrahydrofuran; mineral oil Inert atmosphere; | 90% |
| With sodium hydride; 18-crown-6 ether 1.) THF, 0 degC, 1 h, and 30 min, RT, 2.) 0 degC, 1 h and 50 degC, overnight, THF; Yield given. Multistep reaction; |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
126090-32-2
N-methyl-N-(phenylmethyl)-L-phenylalaninamide hydrochloride

-
-
131949-55-8
Boc-(2S,4R)Hyp-Phe-NMeBzl

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol In dichloromethane Ambient temperature; | 100% |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
109384-24-9
tert-butyl (2S,4R)-2-(aminocarbonyl)-4-hydroxypyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With 1-hydroxybenzotriazol-hydrate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In acetonitrile at 20℃; for 6h; Stage #2: With ammonium hydroxide In acetonitrile at 0 - 20℃; for 1.5h; | 100% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With chloroformic acid ethyl ester; triethylamine In tetrahydrofuran at -15℃; for 0.166667h; Inert atmosphere; Stage #2: With ammonia In tetrahydrofuran; water at -15 - 5℃; for 2h; | 86% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With chloroformic acid ethyl ester; triethylamine In tetrahydrofuran at -10℃; for 1h; Stage #2: With ammonia In tetrahydrofuran; water at -5 - 20℃; for 2h; | 59% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With 1-hydroxybenzotriazol-hydrate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In acetonitrile at 20℃; Stage #2: With ammonium hydroxide In acetonitrile | |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane; N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: With ammonium chloride In dichloromethane; N,N-dimethyl-formamide at 20℃; for 2h; |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
74844-91-0
1-tert-butyl 2-methyl (2S,4R)-4-hydroxy-1,2-pyrrolidinedicarboxylate

| Conditions | Yield |
|---|---|
| In methanol at 0℃; for 1h; | 100% |
| In methanol; hexane; benzene at 20℃; for 1h; | |
| In tetrahydrofuran; methanol; toluene at -78 - 23℃; for 0.166667h; Inert atmosphere; |
-
-
884538-46-9
7-chloro-5-pyridin-2-ylthieno[3,2-b]pyridine

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
884538-47-0
(2S,4R)-1-(tert-butoxycarbonyl)-4-(5-(pyridin-2-yl)thieno[3,2-b]pyridin-7-yloxy)pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In dimethyl sulfoxide at 20℃; for 70h; | 100% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With potassium tert-butylate In dimethyl sulfoxide for 2h; Stage #2: 7-chloro-5-pyridin-2-ylthieno[3,2-b]pyridine In dimethyl sulfoxide at 30℃; for 90h; Stage #3: With hydrogenchloride In water pH=4.5; | 57% |
-
-
132997-77-4
1-chloro-6-methoxyisoquinoline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
630421-67-9
(2S,4R)-1-(tert-butoxycarbonyl)-4-((6-methoxyisoquinolin-1-yl)oxy)pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With potassium tert-butylate In dimethyl sulfoxide at 20℃; for 2h; Stage #2: 1-chloro-6-methoxyisoquinoline In dimethyl sulfoxide at 20℃; for 12h; | 100% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With potassium tert-butylate In dimethyl sulfoxide at 20℃; for 0.5h; Stage #2: 1-chloro-6-methoxyisoquinoline In dimethyl sulfoxide at 20℃; for 12h; | 99% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With potassium tert-butylate In dimethyl sulfoxide at 10 - 20℃; for 0.5h; Stage #2: 1-chloro-6-methoxyisoquinoline In dimethyl sulfoxide at 20℃; for 12h; | 99% |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
106-95-6
allyl bromide

-
-
93962-39-1
(2S,4R)-4-(but-3-en-1-yloxy)-1-(tert-butoxycarbonyl)pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With sodium hydride In tetrahydrofuran; dimethyl sulfoxide; mineral oil at -20 - 0℃; Inert atmosphere; Stage #2: allyl bromide In tetrahydrofuran; dimethyl sulfoxide; mineral oil at -20 - 25℃; for 6h; Inert atmosphere; chemoselective reaction; | 100% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid; allyl bromide With sodium hydride In N,N-dimethyl-formamide at -25 - 20℃; Stage #2: With sodium hydroxide In methanol at 0 - 20℃; | 67% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With sodium hydroxide In tetrahydrofuran; mineral oil at 20℃; for 1h; Inert atmosphere; Stage #2: allyl bromide With 18-crown-6 ether In tetrahydrofuran; mineral oil at 20 - 50℃; |
-
-
7742-73-6
1,3-dichloroisoquinoline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
630425-54-6
C19H21ClN2O5

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With sodium hydride In DMF (N,N-dimethyl-formamide) at 0℃; for 0.5h; Stage #2: 1,3-dichloroisoquinoline In DMF (N,N-dimethyl-formamide) at 20℃; for 12h; | 99.8% |
-
-
1448189-30-7
1-[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methaneamine

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
1448191-54-5
tert‐butyl (2S,4R)‐4‐hydroxy‐2‐({[4‐(4‐methyl‐1,3‐thiazol‐5‐yl)phenyl]methyl}carbamoyl)pyrrolidine‐1‐carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With triethylamine In N,N-dimethyl-formamide at 0℃; for 0.0833333h; Stage #2: 1-[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methaneamine In N,N-dimethyl-formamide at 20℃; | 99.49% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane; N,N-dimethyl-formamide for 15h; | 98% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide for 15h; | 98% |
-
-
853269-47-3
1-ethyl-cyclopropane-sulfonic acid [1(R)-amino-2(S)-vinyl-cyclopropanecarbonyl]-amide hydrochloride

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
853269-48-4
2(S)-[1(R)-(1-ethyl-cyclopropanesulfonylaminocarbonyl)-2(S)-vinyl-cyclopropylcarbamoyl]-4(R)-hydroxy-N-Boc-pyrrolidone

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; bromo-tris(1-pyrrolidinyl)phosphonium hexafluorophosphate In dichloromethane at 20℃; for 1h; | 99% |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
1794-48-5
1-bromo-3-phenylprop-2-yne

-
-
872496-90-7
C19H23NO5

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With sodium hydride at 20℃; for 1h; Stage #2: 1-bromo-3-phenylprop-2-yne for 16h; Heating / reflux; | 99% |
-
-
67-56-1
methanol

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
40216-83-9
L-4-hydroxyproline methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 20℃; for 12h; | 99% |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
589-15-1
1-bromomethyl-4-bromobenzene

-
-
1374041-49-2
C17H22BrNO5

| Conditions | Yield |
|---|---|
| With caesium carbonate In 1-methyl-pyrrolidin-2-one at 0 - 20℃; | 99% |
| With caesium carbonate In 1-methyl-pyrrolidin-2-one at 0 - 20℃; | 99% |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
103-67-3
benzyl-methyl-amine

-
-
330583-66-9
(2S,4R)-2-(Benzyl-methyl-carbamoyl)-4-hydroxy-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 6h; | 98% |
-
-
189816-05-5
4-chloro-7-methoxy-2-phenylquinoline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
259214-37-4
(2S,4R)-1-(tert-butoxycarbonyl)-4-(7-methoxy-2-phenylquinolin-4-yloxy)pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With potassium tert-butylate In dimethyl sulfoxide at 0℃; for 1.5h; Stage #2: 4-chloro-7-methoxy-2-phenylquinoline In dimethyl sulfoxide for 25h; | 98% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With potassium tert-butylate In dimethyl sulfoxide at 0℃; for 1.5h; Stage #2: 4-chloro-7-methoxy-2-phenylquinoline In dimethyl sulfoxide for 25h; | 98% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With potassium tert-butylate In dimethyl sulfoxide at 0℃; for 1.5h; Stage #2: 4-chloro-7-methoxy-2-phenylquinoline In dimethyl sulfoxide for 24h; Stage #3: pH=4.6; Acidic aqueous solution; | 98% |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
74-88-4
methyl iodide

-
-
1374161-77-9
(2S,4R)-4-(tert-butyldimethylsilanyloxy)-2-methylpyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid; methyl iodide With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; for 5h; Stage #2: tert-butyldimethylsilyl chloride With 1H-imidazole In N,N-dimethyl-formamide at 0 - 20℃; for 2h; | 98% |

-
-
1448189-30-7
1-[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methaneamine

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide for 0.0833333h; Stage #2: [4-(4-methyl-1,3-thiazol-5-yl)phenyl]methanamine In N,N-dimethyl-formamide for 15h; | 98% |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
87250-97-3, 113775-22-7
2’-methyl-2’-propanyl (1S,4S)-3-oxo-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 20h; Mitsunobu reaction; | 97% |
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 20℃; for 20h; Mitsunobu Displacement; Inert atmosphere; | 87% |
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran | 82% |
-
-
746657-36-3
(1R,2S)-ethyl 1-amino-2-vinylcyclopropanecarboxylate

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
862119-82-2
(2S,4R)-tert-butyl 2-((1R,2S)-1-(ethoxycarbonyl)-2-vinylcyclopropylcarbamoyl)-4-hydroxypyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 0 - 20℃; | 97% |
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 0 - 20℃; | 96% |
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 0 - 20℃; | 96% |
-
-
1159609-95-6
(1R,2S)-1-amino-2-vinylcyclopropanecarboxylic acid ethyl ester tosylate salt

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
862119-82-2
(2S,4R)-tert-butyl 2-((1R,2S)-1-(ethoxycarbonyl)-2-vinylcyclopropylcarbamoyl)-4-hydroxypyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 0 - 20℃; for 4.5h; | 97% |
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 0 - 20℃; | 89% |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
1357471-22-7
trans-4-tert-butyldiphenylsiloxy-N-Boc-L-proline carboxylic acid

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 50℃; for 4h; Reflux; | 97% |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
61478-26-0
tert-butyl (2S,4R)-4-hydroxy-2-hydroxymethyl-pyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With borane-THF In tetrahydrofuran at 0 - 20℃; for 4.25h; Stage #2: With methanol In tetrahydrofuran at 20℃; for 62h; | 96% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With borane-THF In tetrahydrofuran at 0 - 20℃; for 4.25h; Stage #2: With methanol In tetrahydrofuran at 0 - 20℃; for 62h; | 96% |
| With borane-THF at 0℃; for 1h; Inert atmosphere; | 96% |
-
-
19493-44-8
1-chloroisoquinoline

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
630421-77-1
4-(isoquinoline-1-yloxy)-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1-chloroisoquinoline; (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With potassium tert-butylate In dimethyl sulfoxide at 0 - 20℃; for 25.7167h; Stage #2: Stage #3: With hydrogenchloride In water | 96% |
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With potassium tert-butylate In dimethyl sulfoxide at 10 - 20℃; for 0.5h; Stage #2: 1-chloroisoquinoline In dimethyl sulfoxide at 20℃; for 12h; Stage #3: With citric acid In water; dimethyl sulfoxide at 0℃; Product distribution / selectivity; | 92% |

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
862119-82-2
(2S,4R)-tert-butyl 2-((1R,2S)-1-(ethoxycarbonyl)-2-vinylcyclopropylcarbamoyl)-4-hydroxypyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 0 - 20℃; Product distribution / selectivity; | 96% |
| With 4-methyl-morpholine; HATU In dichloromethane at 20℃; for 18h; | 94% |
| With 4-methyl-morpholine; HATU In dichloromethane at 20℃; for 18h; | 94% |
-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

-
-
107-08-4
1-iodo-propane

-
-
146129-07-9
(2S,4R)-1-(tert-butoxycarbonyl)-4-propoxypyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With sodium hydride In tetrahydrofuran; dimethyl sulfoxide; mineral oil at -20 - 0℃; Inert atmosphere; Stage #2: 1-iodo-propane In tetrahydrofuran; dimethyl sulfoxide; mineral oil at -20 - 25℃; for 24h; Inert atmosphere; chemoselective reaction; | 96% |
-
-
13368-25-7
1-(bromomethyl)-4-vinylbenzene

-
-
13726-69-7
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid With sodium hydride In tetrahydrofuran; dimethyl sulfoxide; mineral oil at -20 - 0℃; Inert atmosphere; Stage #2: 1-(bromomethyl)-4-vinylbenzene In tetrahydrofuran; dimethyl sulfoxide; mineral oil at -20 - 25℃; for 24h; Inert atmosphere; chemoselective reaction; | 96% |
Boc-L-Hydroxyproline Chemical Properties
Molecular structure of Boc-L-Hydroxyproline (CAS NO.13726-69-7) is:
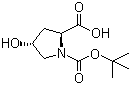
Product Name: Boc-L-Hydroxyproline
CAS Registry Number: 13726-69-7
IUPAC Name: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid
Molecular Weight: 231.24568 [g/mol]
Molecular Formula: C10H17NO5
XLogP3-AA: 0.3
H-Bond Donor: 2
H-Bond Acceptor: 5
Melting Point: 123-127 °C(lit.)
Surface Tension: 57 dyne/cm
Density: 1.312 g/cm3
Flash Point: 190.2 °C
Enthalpy of Vaporization: 74.05 kJ/mol
Boiling Point: 390.9 °C at 760 mmHg
Vapour Pressure: 9.99E-08 mmHg at 25°C
Product Categories: Aminoacids derivatives;Pyrrole&Pyrrolidine&Pyrroline;Amino Acids;Hydroxyproline [Hyp];Unusual Amino Acids;Amino Acids (N-Protected);Biochemistry;Boc-Amino Acids;Boc-Amino acid series
Boc-L-Hydroxyproline Safety Profile
Safety Statements: 22-24/25
S22:Do not breathe dust.
S24/25:Avoid contact with skin and eyes.
WGK Germany: 3
HazardClass: IRRITANT
Boc-L-Hydroxyproline Specification
Boc-L-Hydroxyproline , its cas register number is 13726-69-7. It also can be called trans-N-(tert-Butoxycarbonyl)-4-hydroxy-L-proline ; Boc-Hyp-OH ; (2S,4R)-1-tert-Butyl 4-hydroxy-1,2-pyrrolidinecarboxylate ; 1,2-Pyrrolidinedicarboxylic acid, 4-hydroxy-, 1-(1,1-dimethylethyl) ester, (2S,4R)- .
Related Products
- Boc-L-Hydroxyproline
- 13726-76-6
- 137-26-8
- 13726-84-6
- 13726-85-7
- 137275-81-1
- 137278-09-2
- 137280-55-8
- 137281-08-4
- 137281-23-3
- 137281-39-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View