-
Name
CINOXACIN
- EINECS
- CAS No. 28657-80-9
- Article Data3
- CAS DataBase
- Density 1.64g/cm3
- Solubility
- Melting Point 261-262° (dec)
- Formula C12H10 N2 O5
- Boiling Point 517.2°Cat760mmHg
- Molecular Weight 262.222
- Flash Point 266.6°C
- Transport Information
- Appearance
- Safety Moderately toxic by ingestion, subcutaneous and intravenous routes. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1-Ethyl-3-carboxy-6,7-methylenedioxy-4-cinnolone;Cinobac; Cinobactin; Cinoxacin; Compound 64716; NSC 304467; Noxigram; Uronorm
- PSA 90.65000
- LogP 0.84330
Synthetic route
-
-
74-96-4
ethyl bromide

-
-
90447-46-4
6,7-methylenedioxy-4(1H)-oxo-cinnolin-3-carboxylic acid ethylester

-
-
5137-55-3
Aliquat 336

-
-
28657-80-9
cinoxacin

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium carbonate In water; toluene |

-
-
28657-80-9
cinoxacin

-
-
64-17-5
ethanol

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 2h; Reflux; | 92.9% |

-
-
28657-80-9
cinoxacin

-
-
67-68-5
dimethyl sulfoxide


-
B
-
151933-03-8
{Ni(1-ethyl-1,4-dihydro-4-oxo(1,3)dioxolo(4,5-g)cinnoline-3-carboxylate)2(H2O)2}

| Conditions | Yield |
|---|---|
| With NaOH; H2O In dimethyl sulfoxide mixing DMSO soln. of cinoxacin and of the Ni salt, slow addn. of 5 ml of aq. NaOH with stirring, crystn. of Ni(cinoxacinate)2*2H2O after 1 d; filtration, washing (water), drying at 90°C, crystn. of the Ni complex contg. dimethyl sulfoxide ligands after 2-3 months from the remaining soln., filtration, drying at room temp., elem. anal.; | A 10% B 80% |

-
-
28657-80-9
cinoxacin

-
-
95-14-7
1,2,3-Benzotriazole

-
-
1173208-74-6
3-(1H-benzo[d][1,2,3]triazole-1-carbonyl)-1-ethyl-[1,3]dioxolo[4,5-g]cinnolin-4(1H)-one

| Conditions | Yield |
|---|---|
| Stage #1: 1,2,3-Benzotriazole With thionyl chloride In dichloromethane at 25℃; for 0.5h; Stage #2: cinoxacin In dichloromethane at 25℃; for 2h; | 80% |

| Conditions | Yield |
|---|---|
| With NaOH; H2O In dimethyl sulfoxide mixing of a DMSO soln. of cinoxacin and of ZnCl2, addn. of 1 ml of aq. NaOH with stirring, pptn. of Zn(cinoxacinate)2*4H2O after 12-14 d; filtration, washing (water), drying at 90°C, remaining soln. standing at room temp., crystn. of the Zn complex (contg. additionally DMSO ligands) after a few d, filtration, drying at room temp., elem. anal.; | A 75% B 6% |

-
-
28657-80-9
cinoxacin

-
-
1429439-48-4
1-ethyl-5-nitro-4-oxo-1,4-dihydro-[1,3]dioxolo[4,5-g]cinnolin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; potassium nitrate at 0 - 20℃; for 13h; Inert atmosphere; | 75% |
-
-
28657-80-9
cinoxacin

-
-
67-56-1
methanol

-
-
1429438-89-0
methyl 1-ethyl-6,7-dihydroxy-4-oxo-1,4-dihydrocinnolin-3-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: cinoxacin With boron tribromide In dichloromethane at -30 - 20℃; for 41h; Inert atmosphere; Stage #2: methanol In dichloromethane at -30 - 20℃; Inert atmosphere; | 74.5% |
-
-
28657-80-9
cinoxacin

-
A
-
109773-20-8
1-Ethyl-1H-[1,3]dioxolo[4,5-g]cinnolin-4-one

| Conditions | Yield |
|---|---|
| With oxygen In methanol for 12h; Ambient temperature; Irradiation; in PBS-solution (pH=8); Yields of byproduct given; | A n/a B 62% |
| With oxygen In methanol for 12h; Mechanism; Product distribution; Ambient temperature; Irradiation; Iin PBS-solution (pH=8); | |
| With oxygen In methanol for 12h; Ambient temperature; Irradiation; in PBS-solution (pH=8); Yields of byproduct given; | A 0.580 mol B n/a |
-
-
28657-80-9
cinoxacin

-
-
52462-29-0
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2

-
-
7732-18-5
water

-
-
108-88-3
toluene

| Conditions | Yield |
|---|---|
| With NaOCH3 In methanol; chloroform byproducts: NaCl; addn. of sodium methylate to soln. of ruthenium compd. and sodium nalidixicate in CHCl3/methanol (1/1), refluxing for 6 h; filtration through celite, addn. of toluene, keeping at room temp. with slow evapn. for 3 d, isolation of crystals, washing with hexane, elem. anal.; | 55% |

-
-
28657-80-9
cinoxacin

-
-
28657-79-6
1-ethyl-1,4-dihydro-4-oxo[1,3]dioxolo[4,5-g]cinnoline-3-carbonitrile

| Conditions | Yield |
|---|---|
| Stage #1: cinoxacin With polyphosphate ester; ammonia In chloroform at 119 - 123℃; for 24h; Stage #2: With polyphosphate ester at 120℃; for 1h; | 21% |
-
-
28657-80-9
cinoxacin

-
-
67-68-5
dimethyl sulfoxide

| Conditions | Yield |
|---|---|
| With NaOH In dimethyl sulfoxide mixing DMSO solns. of cinoxacin (0.4 mmol) and CdCl2*2.5H2O (0.1 mmol); slow addn. of NaOH (0.1 M) with continuous stirring;; slow evapn. at room temp.; crystn. after 2-3 mo; elem. anal.;; | 12% |

-
-
28657-80-9
cinoxacin

-
-
67124-17-8
5-ethyl-5H-[1,3]dioxolo[4,5-f]indole-6,7-dione

| Conditions | Yield |
|---|---|
| In formic acid; water (electrochemical reduction); |
-
-
28657-80-9
cinoxacin

-
-
156-57-0
2-mercaptoethylamine hydrochloride

-
-
86222-61-9
1-Ethyl-N-(2-mercaptoethyl)-6,7-methylene-dioxy-4(1H)-oxocinnoline-3-carboxamide

| Conditions | Yield |
|---|---|
| With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide |
-
-
28657-80-9
cinoxacin

-
-
86222-62-0
1-Ethyl-6,7-methylenedioxy-4(1H)-oxocinnoline-3-carboxylic acid, methyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In methanol; water |
-
-
28657-80-9
cinoxacin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; dimethyl sulfoxide Co(ClO4)2*6H2O mixed with cinoxacin; soln. NaOH added with stirring for1 h; filtered; washed (DMSO); dreid in vac. at 50°C to const. wt.; elem. anal.; powd. XRD; TG-DTA; |
-
-
28657-80-9
cinoxacin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; dimethyl sulfoxide cinoxacin mixed with Cu(NO3)2*3H2O; NaOH added with stirring; ppt. filtered; washed (DMSO); dried in vac. at 50°C; elem. anal.; powd. XRD, TG-DTA; |
-
-
28657-80-9
cinoxacin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water cinoxacin mixed with Ni(ClO4)2*6H2O; NaOH added with stirring; evapn. at room temp. for several d; filtered; washed (methanol); dried in vac. at 50°C; elem. anal.; powd. XRD, TG-DTA; |

| Conditions | Yield |
|---|---|
| With NaOH In ethanol; water addn. of freshly prepared soln. of histamine in ethanol to aq. soln. of copper salt, addn. of an aq. soln. of cinoxacin with NaOH; cooling overnight, filtration, cooling filtrate in refrigerator for 7- 8months; elem. anal.; |

-
-
28657-80-9
cinoxacin

| Conditions | Yield |
|---|---|
| In methanol stoich. amts., warm solvent; pptn. on standing for few d, collection (filtration); single crystals fro mother liquor on slow evapn.; |

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide |
-
-
28657-80-9
cinoxacin

-
-
1429438-94-7
1-ethyl-6,7-bis((4-methoxybenzyl)oxy)-3-(pyrrolidin-1-ylmethyl)cinnolin-4(1H)-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: boron tribromide / dichloromethane / 41 h / -30 - 20 °C / Inert atmosphere 1.2: -30 - 20 °C / Inert atmosphere 2.1: potassium carbonate; potassium iodide / acetone / Inert atmosphere; Reflux 3.1: potassium hydroxide / methanol; water / 1.5 h / Inert atmosphere; Reflux 3.2: 20 °C / pH 1 / Inert atmosphere 4.1: chloroformic acid ethyl ester; triethylamine / tetrahydrofuran / 2 h / 20 °C / Inert atmosphere 4.2: 2 h / 20 °C / Inert atmosphere 5.1: Dess-Martin periodane / dichloromethane / 1 h / 20 °C / Inert atmosphere 6.1: sodium tris(acetoxy)borohydride; acetic acid / 1,2-dichloro-ethane / 1.5 h / 20 °C / Inert atmosphere View Scheme |
-
-
28657-80-9
cinoxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: boron tribromide / dichloromethane / 41 h / -30 - 20 °C / Inert atmosphere 1.2: -30 - 20 °C / Inert atmosphere 2.1: potassium carbonate; potassium iodide / acetone / Inert atmosphere; Reflux 3.1: potassium hydroxide / methanol; water / 1.5 h / Inert atmosphere; Reflux 3.2: 20 °C / pH 1 / Inert atmosphere 4.1: thionyl chloride / dichloromethane / 1 h / Inert atmosphere; Reflux View Scheme |
-
-
28657-80-9
cinoxacin

-
-
1429438-99-2
1-ethyl-6,7-bis((4-methoxybenzyl)oxy)-4-oxo-N-(2-(pyrrolidin-1-yl)ethyl)-1,4-dihydrocinnolin-3-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: boron tribromide / dichloromethane / 41 h / -30 - 20 °C / Inert atmosphere 1.2: -30 - 20 °C / Inert atmosphere 2.1: potassium carbonate; potassium iodide / acetone / Inert atmosphere; Reflux 3.1: potassium hydroxide / methanol; water / 1.5 h / Inert atmosphere; Reflux 3.2: 20 °C / pH 1 / Inert atmosphere 4.1: thionyl chloride / dichloromethane / 1 h / Inert atmosphere; Reflux 5.1: triethylamine / dichloromethane / 0.5 h / 20 °C / Cooling with ice View Scheme |
-
-
28657-80-9
cinoxacin

-
-
1429439-01-9
1-ethyl-6,7-bis((4-methoxybenzyl)oxy)-3-(piperidin-1-ylmethyl)cinnolin-4(1H)-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: boron tribromide / dichloromethane / 41 h / -30 - 20 °C / Inert atmosphere 1.2: -30 - 20 °C / Inert atmosphere 2.1: potassium carbonate; potassium iodide / acetone / Inert atmosphere; Reflux 3.1: potassium hydroxide / methanol; water / 1.5 h / Inert atmosphere; Reflux 3.2: 20 °C / pH 1 / Inert atmosphere 4.1: chloroformic acid ethyl ester; triethylamine / tetrahydrofuran / 2 h / 20 °C / Inert atmosphere 4.2: 2 h / 20 °C / Inert atmosphere 5.1: methanesulfonyl chloride; triethylamine / dichloromethane / 2 h / Cooling with ice; Inert atmosphere 5.2: Inert atmosphere View Scheme |
-
-
28657-80-9
cinoxacin

-
-
1429439-49-5
5-amino-1-ethyl-4-oxo-1,4-dihydro-[1,3]dioxolo[4,5-g]cinnolin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sulfuric acid; potassium nitrate / 13 h / 0 - 20 °C / Inert atmosphere 2: hydrogenchloride; palladium on activated charcoal; hydrogen; acetic acid / 5 h / 2585.81 Torr / Inert atmosphere View Scheme |
-
-
28657-80-9
cinoxacin

-
-
1429439-50-8
3-carboxy-1-ethyl-4-oxo-1,4-dihydro-[1,3]dioxolo[4,5-g]cinnolin-5-diazonium chloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sulfuric acid; potassium nitrate / 13 h / 0 - 20 °C / Inert atmosphere 2: hydrogenchloride; palladium on activated charcoal; hydrogen; acetic acid / 5 h / 2585.81 Torr / Inert atmosphere 3: hydrogenchloride; sodium nitrite / water / 5 h / 45 °C / Inert atmosphere View Scheme |
-
-
28657-80-9
cinoxacin

-
-
1429439-51-9
5-chloro-1-ethyl-6,7-dihydroxy-4-oxo-1,4-dihydrocinnolin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sulfuric acid; potassium nitrate / 13 h / 0 - 20 °C / Inert atmosphere 2: hydrogenchloride; palladium on activated charcoal; hydrogen; acetic acid / 5 h / 2585.81 Torr / Inert atmosphere 3: hydrogenchloride; sodium nitrite / water / 5 h / 45 °C / Inert atmosphere 4: sulfuric acid / 20 h / 95 °C / Inert atmosphere View Scheme |
CINOXACIN Chemical Properties
Product Name: Cinoxacin (CAS NO.28657-80-9)
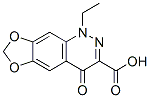
Molecular Formula: C12H10N2O5
Molecular Weight: 262.22g/mol
Mol File: 28657-80-9.mol
Einecs: 249-133-8
Appearance: Light tan crystals
Boiling point: 517.2 °C at 760 mmHg
Density: 1.64 g/cm3
Surface Tension: 65.7 dyne/cm
Enthalpy of Vaporization: 83.11 kJ/mol
Vapour Pressure: 1.61E-11 mmHg at 25°C
XLogP3-AA: 2.1
H-Bond Donor: 1
H-Bond Acceptor: 7
Product Categories: Interferes with DNA SynthesisAntibiotics; Quinolones and FluoroquinolonesMore...Close...; A - KAntibiotics; Antibacterial; Antibiotics A to; Antibiotics A-FAntibiotics; Antibiotics by Application; Antineoplastic and Immunosuppressive AntibioticsAntibiotics; Chemical Structure Class; Inhibits an EnzymeAntibiotics; Mechanism of Action; Spectrum of Activity
CINOXACIN Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 850mg/kg (850mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Chemotherapy Vol. 28(Suppl, |
| mouse | LD50 | oral | 2330mg/kg (2330mg/kg) | SENSE ORGANS AND SPECIAL SENSES: MYDRIASIS (PUPILLARY DILATION): EYE BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Chemotherapy Vol. 28(Suppl, |
| mouse | LD50 | subcutaneous | 900mg/kg (900mg/kg) | SENSE ORGANS AND SPECIAL SENSES: MYDRIASIS (PUPILLARY DILATION): EYE BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Chemotherapy Vol. 28(Suppl, |
| rat | LD50 | intravenous | 860mg/kg (860mg/kg) | SENSE ORGANS AND SPECIAL SENSES: MYDRIASIS (PUPILLARY DILATION): EYE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Chemotherapy Vol. 28(Suppl, |
| rat | LD50 | oral | 3610mg/kg (3610mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Chemotherapy Vol. 28(Suppl, |
| rat | LD50 | subcutaneous | 1380mg/kg (1380mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Chemotherapy Vol. 28(Suppl, |
CINOXACIN Safety Profile
Moderately toxic by ingestion, subcutaneous and intravenous routes. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx.
CINOXACIN Specification
Cinoxacin ,its CAS NO. is 28657-80-9,the synonyms is 1-Ethyl-1,4-dihydro-4-oxo[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid;cinoxacin ; Timtec-bb sbb003082 ; 1-Ethyl-6,7-methylenedioxy-4(1h)-oxocinnoline-3-carboxylicacid ; 3)Dioxolo(4,5-g)cinnoline-3-carboxylicacid,1,4-dihydro-1-ethyl-4-oxo-( ; Cinobac ; 1-Ethyl-4-oxo-1,4-dihydro-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View