-
Name
Caffeic acid
- EINECS 206-361-2
- CAS No. 331-39-5
- Article Data165
- CAS DataBase
- Density 1.478 g/cm3
- Solubility soluble in hot water
- Melting Point 211-213 °C (dec.)(lit.)
- Formula C9H8O4
- Boiling Point 416.8 °C at 760 mmHg
- Molecular Weight 180.16
- Flash Point 220 °C
- Transport Information
- Appearance Light yellow to greenish-yellow powder
- Safety 26-36/37/39-36
- Risk Codes 36/37/38-40-63-68
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Cinnamicacid, 3,4-dihydroxy- (8CI);3,4-Dihydroxybenzeneacrylic acid;3,4-Dihydroxycinnamic acid;3-(3,4-Dihydroxyphenyl)-2-propenoic acid;3-(3,4-Dihydroxyphenyl)propenoic acid;4-(2-Carboxyethenyl)-1,2-dihydroxybenzene;4-(2'-Carboxyvinyl)-1,2-dihydroxybenzene;NSC 57197;NSC 623438;
- PSA 77.76000
- LogP 1.19560
Synthetic route

| Conditions | Yield |
|---|---|
| With silica gel at 110 - 120℃; for 0.0833333h; Condensation; Solid-phase reaction; Decarboxylation; microwave irradiation; | 76% |
| With pyridine; aniline In toluene at 95℃; | 55.7% |
| With pyridine; aniline In toluene for 2h; Reflux; | 44.2% |

| Conditions | Yield |
|---|---|
| With sodium dihydrogenphosphate at 25℃; for 0.25h; pH=7; Reagent/catalyst; Glovebox; | 50% |
| With dipotassium hydrogenphosphate; iron(II) sulfate; citric acid at 30℃; for 1h; Product distribution; pH 5.0, investigated effects of various metal ions and various compounds and enzymes; | |
| With leaves Silene dioica at 30℃; for 0.166667h; Mechanism; incubation with glucose-6-posphate and NADP; |
-
-
40918-96-5
methyl (2E)-3-(1,3-benzodioxol-5-yl)acrylate

-
-
331-39-5
caffeic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl (2E)-3-(1,3-benzodioxol-5-yl)acrylate With boron tribromide In chloroform at 25℃; for 16h; Stage #2: With sodium hydrogencarbonate In chloroform; water at 25℃; pH=7; | 38.3% |

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) perchlorate; ascorbic acid In water | 24% |
| In various solvent(s) at 37℃; for 1h; Product distribution; Mechanism; air, phenobarbital-induced rat liver microsomes NADPH, pH = 7.4; other enzymatic system; |
-
-
140-10-3
(E)-3-phenylacrylic acid

-
A
-
13080-39-2
1-[3-(4-hydroxyphenyl)-2-propenoate]-β-D-glucopyranoside

-
B
-
331-39-5
caffeic acid

-
C
-
7400-08-0
p-Coumaric Acid

| Conditions | Yield |
|---|---|
| In ethanol; water at 25℃; for 72h; cell culture of Nicotiana tabacum; |

-
-
1049703-62-9
chlorogenic acid

-
A
-
331-39-5
caffeic acid

-
B
-
77-95-2, 1010-25-9, 7216-27-5, 23804-29-7, 23804-41-3, 23804-44-6, 36413-60-2, 135558-83-7, 135558-85-9, 470-63-3, 562-73-2, 23804-34-4, 23804-38-8
1,3,4,5-tetrahydroxy-cyclohexanecarboxylic acid

-
C
-
2450-53-5, 16758-05-7, 89919-61-9, 89919-62-0, 101222-97-3
3,5-dicaffeoylquinic acid

| Conditions | Yield |
|---|---|
| at 30℃; for 0.5h; pH 6.4; enzyme from Ipomoea batatas; |

-
-
92279-06-6
3-(3',4'-dioxo-1',5'-cyclohexadienyl)propenoic acid

-
-
873-55-2
sodium benzenesulfonate

-
A
-
331-39-5
caffeic acid

-
B
-
58058-71-2
6'-phenysulfonylcaffeic acid

-
C
-
58058-69-8
5'-phenysulfonylcaffeic acid

| Conditions | Yield |
|---|---|
| With O at 30℃; for 48h; alkaline solution, pH 10.0; |

-
-
202650-88-2
3-caffeoylquinic acid

-
-
331-39-5
caffeic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol for 4h; Heating; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 90℃; for 2h; Product distribution; study of the acid hydrolysis; |
-
-
104777-68-6
3,4-dihydroxy-β-phenethyl-O-β-D-glucopyranosyl-(1->3)-4-O-caffeoyl-β-D-glucopyranoside

-
-
331-39-5
caffeic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 50℃; for 1h; Product distribution; |
-
-
97747-56-3
cis-verbascoside

-
A
-
2280-44-6
D-Glucose

-
B
-
73-34-7
L-rhamnose

-
C
-
331-39-5
caffeic acid

-
D
-
10597-60-1
hydroxytyrosol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium hydroxide 1.) MeOH, H2O, reflux, 3 h; 2.) MeOH, H2O, reflux; |

-
-
110065-28-6
methyl 2,5-di-O-caffeyl-α-L-arabinofuranoside

-
A
-
331-39-5
caffeic acid

-
B
-
3795-69-5
methyl β-L-arabinofuranoside

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol Product distribution; |
-
-
61276-17-3, 97747-56-3, 138604-99-6, 145306-71-4
verbascoside

-
A
-
331-39-5
caffeic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol for 4h; Ambient temperature; | A 51 mg B 195 mg |
-
-
2450-53-5, 16758-05-7, 89919-61-9, 89919-62-0, 101222-97-3
3,5-dicaffeoylquinic acid

-
A
-
331-39-5
caffeic acid

-
B
-
77-95-2
D-(-)-quinic acid

| Conditions | Yield |
|---|---|
| With water; unspecific esterase hydrolysis; | |
| With water hydrolysis with unspecific esterase; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol Ambient temperature; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol Heating; |

| Conditions | Yield |
|---|---|
| In hydrogenchloride reflux 30 min, room temp. overnight; |
-
-
23284-22-2, 29048-92-8, 56614-74-5
1,6-di-O-caffeoyl-D-glucopyranoside

-
A
-
2280-44-6
D-Glucose

-
B
-
331-39-5
caffeic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol Heating; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 1h; Ambient temperature; |
-
-
79916-77-1
forsythiaside

-
A
-
2280-44-6
D-Glucose

-
B
-
73-34-7
L-rhamnose

-
C
-
331-39-5
caffeic acid

-
D
-
10597-60-1
hydroxytyrosol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide Ambient temperature; hydrolysis; |

-
-
122412-14-0
(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl (E)-3-(3,4-dihydroxyphenyl)acrylate

-
A
-
331-39-5
caffeic acid

-
B
-
970-73-0, 1617-55-6, 3371-27-5, 13425-13-3, 136892-45-0, 970-74-1
epigallocatechin

| Conditions | Yield |
|---|---|
| In water for 2h; Ambient temperature; tannase; |

| Conditions | Yield |
|---|---|
| With hesperidinase In water at 32℃; for 48h; Product distribution; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 90℃; for 2h; Product distribution; study of the acid hydrolysis; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 100℃; for 4h; Title compound not separated from byproducts; |

-
-
331-39-5
caffeic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 50℃; for 1h; Product distribution; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide for 8.5h; Heating; | A n/a B 20 mg |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate In water at 100℃; for 4h; Product distribution; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate In water at 100℃; for 4h; Product distribution; |

-
-
331-39-5
caffeic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate In water at 100℃; for 4h; Product distribution; |

-
A
-
331-39-5
caffeic acid

-
B
-
38681-85-5
5,7-dihydroxy-3-[β-D-glucopyranosyl-(1->2)-β-D-glucopyranosyl-(1->2)-β-D-glucopyranosyl]-2-(3,4-dihydroxyphenyl)-4H-1-benzopyran-4-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In sodium hydroxide for 24h; Product distribution; Ambient temperature; other reagent; |

| Conditions | Yield |
|---|---|
| With Dowex 50W-X8 Heating; | 100% |
| With sulfuric acid for 24h; Reflux; | 100% |
| With sulfuric acid at 70℃; for 1h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 100% |
| With pyridine at 20℃; | 95% |
| With pyridine at 20℃; | 95% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran 1) room temp, 1.5 h, caution: heavy gas production, 2) reflux, 2 h; | 100% |
| In N,N-dimethyl-formamide for 2h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 70℃; for 2h; | 99% |
| With thionyl chloride for 4h; Heating; | 97% |
| With sulfuric acid In water at 82℃; for 24h; Reagent/catalyst; | 96% |

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In methanol at 20℃; under 760.051 Torr; Inert atmosphere; | 99% |
| With palladium 10% on activated carbon; hydrogen In methanol at 25℃; under 2585.81 Torr; for 3h; | 95% |
| With ferrous ammonium sulphate hexahydrate; isopropyl β-D-thiogalactopyranoside In aq. phosphate buffer at 37℃; for 48h; Catalytic behavior; Reagent/catalyst; Concentration; | 92% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In tetrahydrofuran for 7h; Heating; | 99% |
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 6h; Inert atmosphere; | 85% |
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 2.5h; | |
| With dicyclohexyl-carbodiimide In tetrahydrofuran Reflux; |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In tetrahydrofuran for 7h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine In acetonitrile at 20℃; for 4h; Cooling with ice; | 97.1% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In tetrahydrofuran for 7h; Heating; | 97% |
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 7h; Inert atmosphere; | 82% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In tetrahydrofuran for 6h; Heating; | 97% |
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 7h; Inert atmosphere; | 82% |
| With dicyclohexyl-carbodiimide In tetrahydrofuran Reflux; |
-
-
331-39-5
caffeic acid

-
-
725228-49-9
tert-butyl 3-(piperidin-4-yl)benzylcarbamate

-
-
1290056-81-3
(E)-tert-butyl 3-(1-(3-(3,4-dihydroxyphenyl)acryloyl)piperidin-4-yl)benzylcarbamate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 96% |
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; |
-
-
331-39-5
caffeic acid

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
203118-32-5
(E)-3-(3,4-bis((tert-butyldimethylsilyl)oxy)phenyl)acrylic acid

| Conditions | Yield |
|---|---|
| Stage #1: caffeic acid; tert-butyldimethylsilyl chloride With N-ethyl-N,N-diisopropylamine In dichloromethane at 25℃; for 14h; Stage #2: With potassium carbonate In tetrahydrofuran; water for 2h; | 95% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 25℃; for 14h; | 95% |
| With 1H-imidazole In N,N-dimethyl-formamide for 20h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In tetrahydrofuran for 7h; Heating; | 95% |
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 8h; Inert atmosphere; | 78% |
| Stage #1: caffeic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.333333h; Stage #2: aniline With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 16.33h; | 7.06% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 50℃; for 24h; | 95% |
| With sulfuric acid at 90℃; for 2h; Fischer–Speier Esterification; Molecular sieve; |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In tetrahydrofuran for 7h; Heating; | 94% |
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 6h; Inert atmosphere; | 84% |
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 2.5h; |

| Conditions | Yield |
|---|---|
| Stage #1: caffeic acid With sodium hydroxide In water pH=13; Stage #2: dimethyl sulfate In water at 20℃; for 10h; pH=> 10; Stage #3: With hydrogenchloride In water pH=2; | 94% |
| With sodium hydroxide for 6h; | 78.8% |
| With sodium hydroxide In water for 3.5h; Heating; | 78.8% |
-
-
26042-63-7
silver(I) hexafluorophosphate

-
-
200358-34-5, 200259-33-2
dichloro[1,4-bis(diphenylphosphino)butane](2,2'-bipyridine)ruthenium(II)

-
-
331-39-5
caffeic acid

| Conditions | Yield |
|---|---|
| In methanol for 0.333333h; Darkness; Inert atmosphere; | 94% |


| Conditions | Yield |
|---|---|
| With 1-methyl-3-(4-sulfobutyl)-1H-imidazol-3-ium 4-methylbenzene-1-sulfonate at 90℃; under 760.051 Torr; for 2h; Activation energy; Catalytic behavior; Reagent/catalyst; Temperature; Concentration; | 93.8% |

| Conditions | Yield |
|---|---|
| With sulfuric acid under 9308.91 Torr; Microwave irradiation; Inert atmosphere; Sealed tube; Reflux; | 93% |
| Stage #1: caffeic acid With thionyl chloride In 1,4-dioxane for 1h; Heating; Stage #2: propan-1-ol In 1,4-dioxane Heating; Further stages.; | 86% |
| With thionyl chloride at 0 - 50℃; for 16.5h; | 65.2% |

| Conditions | Yield |
|---|---|
| With sulfuric acid under 9308.91 Torr; Microwave irradiation; Inert atmosphere; Sealed tube; Reflux; | 93% |
| Stage #1: caffeic acid With thionyl chloride In 1,4-dioxane for 1h; Heating; Stage #2: butan-1-ol In 1,4-dioxane Heating; Further stages.; | 86% |
| With sulfuric acid for 0.166667h; Microwave irradiation; Heating; | 80% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 92℃; for 0.0666667h; Microwave irradiation; | 93% |
| With sulfuric acid under 9308.91 Torr; Microwave irradiation; Inert atmosphere; Sealed tube; Reflux; | 92% |
| Stage #1: caffeic acid With thionyl chloride In 1,4-dioxane for 1h; Heating; Stage #2: isopropyl alcohol In 1,4-dioxane Heating; Further stages.; | 82% |
| With toluene-4-sulfonic acid In benzene for 144h; Heating; | 23% |
| With toluene-4-sulfonic acid In toluene at 120℃; for 4h; Fischer esterification; |
-
-
331-39-5
caffeic acid

-
-
79-22-1
methyl chloroformate

-
-
861897-00-9
3,4-bis[(methoxycarbonyl)oxy]cinnamic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 0℃; for 1h; | 93% |
| With TEA In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In N,N-dimethyl-formamide at 27℃; for 18h; | 93% |
| Stage #1: caffeic acid; Propargylamine With benzotriazol-1-ol; triethylamine In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Stage #2: With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In N,N-dimethyl-formamide for 18h; | 20% |
-
-
331-39-5
caffeic acid

-
-
14533-84-7
pentafluorophenyl trifloroacetate

-
-
916348-43-1
pentafluorophenyl 3,4-dihydroxycinnamate

| Conditions | Yield |
|---|---|
| With pyridine In N,N-dimethyl-formamide | 93% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 118℃; for 0.0666667h; Microwave irradiation; | 92% |
| With sulfuric acid under 9308.91 Torr; Microwave irradiation; Inert atmosphere; Sealed tube; Reflux; | 91% |
| With toluene-4-sulfonic acid In toluene at 120℃; for 4h; Fischer esterification; |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In tetrahydrofuran for 7h; Heating; | 91% |
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 7h; Inert atmosphere; | 76% |
| With dicyclohexyl-carbodiimide In tetrahydrofuran for 7h; Reflux; | 48% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 142℃; for 0.0833333h; Microwave irradiation; | 91% |
| With sulfuric acid under 9308.91 Torr; Microwave irradiation; Inert atmosphere; Sealed tube; Reflux; | 90% |
| With ytterbium(III) triflate In nitromethane at 120℃; for 1h; | 38.1% |

| Conditions | Yield |
|---|---|
| With sulfuric acid under 9308.91 Torr; Microwave irradiation; Inert atmosphere; Sealed tube; Reflux; | 91% |
| With dicyclohexyl-carbodiimide In 1,4-dioxane at 5℃; for 48h; | |
| With toluene-4-sulfonic acid Reflux; |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; | 91% |
-
-
331-39-5
caffeic acid

-
-
100-39-0
benzyl bromide

-
-
951288-55-4
(E)-benzyl 3-(3,4-bis(benzyloxy)phenyl)acrylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 90% |
| With potassium carbonate In acetone for 15h; Reflux; Inert atmosphere; | 90% |
| With potassium carbonate In acetone for 12h; Heating; | 85% |
| With potassium carbonate In dichloromethane at 20℃; for 20h; | 80% |
Caffeic acid Chemical Properties
Molecular Formula: C9H8O4
Molecular Weight: 180.16 g/mol
EINECS: 206-361-2
Index of Refraction: 1.706
Molar Refractivity: 47.47 cm3
Molar Volume: 121.8 cm3
Surface Tension: 77.8 dyne/cm
Density: 1.478 g/cm3
Flash Point: 220 °C
Enthalpy of Vaporization: 70.65 kJ/mol
Boiling Point: 416.8 °C at 760 mmHg
Vapour Pressure: 1.08E-07 mmHg at 25 °C
Melting point: 34-39 °C
Storage tempreture: Store at RT.
Water solubility: Soluble in hot water
Solubility: Ethanol : 50 mg/mL
Appearance: Light yellow to greenish-yellow powder
Structure of Caffeic acid (CAS NO.331-39-5):
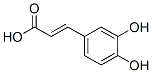
IUPAC Name: (E)-3-(3,4-Dihydroxyphenyl)prop-2-enoic acid
Canonical SMILES: C1=CC(=C(C=C1C=CC(=O)O)O)O
Isomeric SMILES: C1=CC(=C(C=C1/C=C/C(=O)O)O)O
InChI: InChI=1S/C9H8O4/c10-7-3-1-6(5-8(7)11)2-4-9(12)13/h1-5,10-11H,(H,12,13)/b4-2+
InChIKey: QAIPRVGONGVQAS-DUXPYHPUSA-N
Product Category of Caffeic acid (CAS NO.331-39-5): Pharmaceutical Intermediates;Aromatic Cinnamic Acids, Esters and Derivatives;Cinnamic acid;Organic acids;Antioxidant;Biochemistry
Caffeic acid Uses
Caffeic acid (CAS NO.331-39-5) may be the active ingredient in caffenol, a do-it-yourself black and white photographic developer made from instant coffee. It is also used as a matrix in MALDI mass spectroscopy analyses, and is a key intermediate in the biosynthesis of lignin, one of the principal sources of biomass.
Caffeic acid Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | > 721mg/kg (721mg/kg) | Yakugaku Zasshi. Journal of Pharmacy. Vol. 104, Pg. 793, 1984. | |
| rat | LDLo | intraperitoneal | 1500mg/kg (1500mg/kg) | Toxicology and Applied Pharmacology. Vol. 36, Pg. 227, 1976. |
Caffeic acid (CAS NO.331-39-5) hasn't been listed as a carcinogen by NTP, IARC, ACGIH, or CA Prop 65. And the toxicological properties have not been fully investigated. You can see actual entry in RTECS for complete information.
Caffeic acid Safety Profile
Moderately toxic by intraperitoneal route. An experimental teratogen. Mutation data reported. When heated to decomposition it emits acrid smoke and fumes.
Hazard Codes:  Xn,
Xn, Xi
Xi
Risk Statements: 36/37/38-40-63-68
R36/37/38:Irritating to eyes, respiratory system and skin.
R40:Limited evidence of a carcinogenic effect.
R63:Possible risk of harm to the unborn child.
R68:Possible risk of irreversible effects.
Safety Statements: 26-36/37/39-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
Caffeic acid Specification
Caffeic acid , its cas register number is 331-39-5. It also can be called 3,4-Dihydroxycinnamic acid ; 3,4-Dihydroxybenzeneacrylic acid ; and 3-(3,4-Dihydroxy phenyl)-2-propenoic acid . It is a naturally occurring organic compound, and is found in all plants because it is a key intermediate in the biosynthesis of lignin. It is hazardous, so the first aid measures and others should be known. Such as: When on the skin: first, should flush skin with plenty of water immediately for at least 15 minutes while removing contaminated clothing. Secondly, get medical aid. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Then get medical aid soon. While, it's inhaled: Remove from exposure and move to fresh air immediately. Give artificial respiration while not breathing. When breathing is difficult, give oxygen. And as soon as to get medical aid. Then you have the ingesting of the product: Wash mouth out with water, and get medical aid immediately.
In addition, Caffeic acid (CAS NO.331-39-5) could be stable under normal temperatures and pressures. It is not compatible with strong oxidizing agents, strong acids, strong bases, and you must not take it with incompatible materials. And also prevent it to broken down into hazardous decomposition products: Carbon monoxide, carbon dioxide.
Related Products
- Caffeic acid
- 33140-80-6
- 3314-30-5
- 33143-29-2
- 3314-35-0
- 33143-92-9
- 331442-12-7
- 331443-00-6
- 3314-49-6
- 33145-10-7
- 331465-71-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View