-
Name
2-Bornanone
- EINECS 200-945-0
- CAS No. 76-22-2
- Article Data196
- CAS DataBase
- Density 0.982 g/cm3
- Solubility 0.12 g/100 mL (25 °C) in water
- Melting Point 179 °C
- Formula C10H16O
- Boiling Point 207.4 °C at 760 mmHg
- Molecular Weight 152.236
- Flash Point 64.4 °C
- Transport Information UN 2717 4.1/PG 3
- Appearance Colourless solid
- Safety 16-26-37/39
- Risk Codes 11-22-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xn,
Xn,  Xi
Xi
- Synonyms 1,7,7-Trimethylbicyclo[2.2.1]-2-heptanone;1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one;2-Bornanone;Bicyclo(2.2.1)heptan-2-one, 1,7,7-trimethyl-;2-Kamfanon;1,7,7-Trimethylnorcamphor;2-Camphanone;Bornane, 2-oxo-;DL-Camphor;Norcamphor, 1,7,7-trimethyl-;DL-Bornan-2-one;(+-)-Camphor;
- PSA 17.07000
- LogP 2.40170
Synthetic route

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; chromium tetra(tert-butoxide) In benzene at 20℃; for 24h; | 100% |
| With peracetic acid; 2,4-dimethyl-2,4-pentanediol cyclic Cr(VI) ester In tetrachloromethane; dichloromethane at 0℃; for 12h; | 99% |
| With dimethyl sulfoxide; triethylamine; trichloromethyl chloroformate In dichloromethane | 92% |
-
-
124-76-5, 464-43-7, 464-45-9, 507-70-0, 6627-72-1, 10334-13-1, 10385-78-1, 16725-71-6, 24393-70-2
1,7,7-trimethylbicyclo[2.2.1]heptan-2-ol

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; 3-[4'-(diacetoxyiodo)phenoxy]-1-propyl-N,N,N-trimethylammonium 4-methylbenzenesulfonate In dichloromethane at 20℃; for 12h; | 99% |
| With aluminum oxide; sodium bromite In dichloromethane for 24h; Ambient temperature; | 98% |
| With phosphomolybdic acid; oxygen; tetrabutylammonium acetate; palladium diacetate In toluene at 100℃; under 22502.3 Torr; for 16h; Solvent; Time; | 96% |
| With 1-methyl-1H-imidazole; tetrakis(acetonitrile)copper(I) trifluoromethanesulfonate; 4,4'-Dimethoxy-2,2'-bipyridin; 9-azabicyclo[3.3.1]nonane N-oxyl; oxygen In acetonitrile at 70℃; for 1h; | 77% |
| With sodium dichromate; sulfuric acid for 3h; Heating; | 55.6% |
-
-
24393-70-2
isoborneol

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| With bis-trimethylsilanyl peroxide; dipyridinium dichromate In dichloromethane for 0.5h; | 98% |
| With dihydrogen peroxide; methyltrioctylammonium tetrakis(oxodiperoxotungsto)phosphate In 1,1,2,2-tetrachloroethylene at 90℃; for 0.75h; | 95% |
| With oxalyl dichloride; C4F9CH2CH2S(O)CH3 In dichloromethane at -30℃; Swern oxidation; | 94% |

| Conditions | Yield |
|---|---|
| With ammonium iodide; dihydrogen peroxide; acetic acid In water at 20℃; for 1h; | 95% |
-
-
4581-48-0, 10281-41-1, 72059-63-3, 120522-07-8, 121702-12-3, 124815-32-3
bornan-2-one semicarbazone

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromate In ethanol for 3h; Heating; | 92% |
| With potassium permanganate; silica gel In water at 20℃; for 0.5h; | 80% |
-
-
18674-50-5
camphor oxime

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| With dibromamine-B In tetrachloromethane at 20 - 25℃; for 0.333333h; | 90% |
| With N-iodo-succinimide; water In acetone for 0.05h; Microwave irradiation; | 87% |
| With water; oxygen In acetonitrile at 60℃; under 760.051 Torr; for 6h; Autoclave; Green chemistry; | 83% |
| With Oxone; silica gel In water; acetic acid at 25℃; for 24h; | 57% |
-
-
18529-99-2, 75524-60-6
1-bornenyl acetate

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| With water; dichloro bis(acetonitrile) palladium(II) In isopropyl alcohol at 80℃; for 24h; | 87% |
-
-
76-29-9, 1925-58-2, 10293-06-8, 27799-07-1, 55057-87-9, 64474-54-0, 67337-12-6, 107870-25-7
3-bromo-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| With sodium hydrogen telluride In ethyl acetate for 1h; Ambient temperature; | 78% |

| Conditions | Yield |
|---|---|
| With water; zinc In acetonitrile at 80℃; for 11h; Inert atmosphere; Sealed tube; | 76% |
| With Methyltrichlorosilane; sodium iodide In acetonitrile for 18h; Heating; | 60% |

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| Stage #1: 1,7,7-trimethyl-2-nitrobicyclo[2.2.1]heptane With potassium hydride In 1,4-dioxane at 10℃; for 1h; Stage #2: With chloro-trimethyl-silane In 1,4-dioxane for 15h; Nef reaction; Heating; Further stages.; | 62% |

| Conditions | Yield |
|---|---|
| With clay supported cupric nitrate In dichloromethane Ambient temperature; | 30% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; oxygen; rose bengal In chloroform Irradiation; | A 30% B 15% |
| With sodium hydroxide; oxygen; rose bengal In chloroform Rate constant; Mechanism; Irradiation; | A 30% B 15% |

| Conditions | Yield |
|---|---|
| -diethylacetal; |

-
-
56-23-5
tetrachloromethane

-
-
15736-81-9
3-iodo-bornan-2-one

-
-
124-41-4
sodium methylate

-
A
-
6812-07-3, 30543-79-4
4,7,7-trimethyl-3-oxo-norbornane-2-carbaldehyde

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| α-iodo-d-camphor; |

-
-
56-23-5
tetrachloromethane

-
-
6627-72-1
rac-endo-borneol

-
-
76-22-2
Camphor

-
B
-
6053-45-8
2,2-dimethyl-cyclopentane-1,1,3-tricarboxylic acid

| Conditions | Yield |
|---|---|
| bei der Ozonisation; |

-
-
60-29-7
diethyl ether

-
-
124-41-4
sodium methylate

-
A
-
6812-07-3, 30543-79-4
4,7,7-trimethyl-3-oxo-norbornane-2-carbaldehyde

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| α.α'-diiodo-d-camphor; |


| Conditions | Yield |
|---|---|
| With tetrachloromethane; chromic acid | |
| With chromic acid; benzene |

| Conditions | Yield |
|---|---|
| With tetrachloromethane; ozone |

| Conditions | Yield |
|---|---|
| With copper at 135 - 140℃; |

| Conditions | Yield |
|---|---|
| durch Erhitzen der Daempfe mit Sauerstoff oder Luft in Gegenwart von Kupfer; | |
| With methyllithium; calcium carbonate durch Einw. von Metalloxyden oder Superoxyden; | |
| With chlorine |
-
-
736109-58-3, 787517-91-3
1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-one

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| With aluminium trichloride; toluene at 80 - 85℃; Behandeln des Reaktiongemisches mit Wasser; |

| Conditions | Yield |
|---|---|
| With chromic acid; acetic acid at 140℃; <5-oxo-d-bornyl>-acetate; |

| Conditions | Yield |
|---|---|
| With nickel catalysts | |
| With copper |

| Conditions | Yield |
|---|---|
| at 300℃; beim Leiten ueber Kupfer; | |
| Multi-step reaction with 2 steps 1: 145 °C 2: chromic acid; glacial acetic acid / 140 °C View Scheme |
-
-
25611-66-9
4,7,7,4',7',7'-hexamethyl-[2,2']binorbornyl-3,3'-dione

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| at 250℃; isodicamphor; |
-
-
13829-73-7, 27535-95-1, 86707-47-3, 86707-48-4
bornane-2,3-dione-3-hydrazone

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| at 205 - 210℃; α--quinone-hydrazone-(3); |
-
-
134234-71-2
2-carbethoxy-3-oxo-4,7,7-trimethylbicyclo<2,2,1>heptane

-
-
141-52-6
sodium ethanolate

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| at 200℃; im Autoklaven; d-camphocarboxylic acid ethyl ester; |


| Conditions | Yield |
|---|---|
| <3-iodo-d-camphoryl-(3)>-formaldehyde; |

-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| at 160 - 170℃; das Silbersalz reagiert; |

| Conditions | Yield |
|---|---|
| With tetrachloromethane; ozone |
-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| With potassium 9-t-butyl-9-boratabicyclo<3.3.1>nonane In tetrahydrofuran at 0℃; | 99.9% |

| Conditions | Yield |
|---|---|
| zinc trifluoromethanesulfonate In 1,2-dichloro-ethane for 15h; Heating; | 99% |
| With Cu(OTf)2-SiO2 In toluene at 80℃; for 60h; | 91% |
| With silica gel; toluene-4-sulfonic acid In dichloromethane for 24h; Heating; | 86% |
| With silica gel; zirconium(IV) chloride In dichloromethane for 72h; Ambient temperature; | 96 % Chromat. |

| Conditions | Yield |
|---|---|
| 98% |

| Conditions | Yield |
|---|---|
| 98% |

| Conditions | Yield |
|---|---|
| 97% |
-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| With hydrazine hydrate; acetic acid In ethanol for 24h; Reflux; | 97% |
-
-
76-22-2
Camphor

-
-
18674-50-5
camphor oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride In ethanol for 4h; Mechanism; Reflux; | 94% |
| With hydroxylamine hydrochloride; sodium acetate In ethanol at 60℃; for 24h; Inert atmosphere; | 85% |
| With hydroxylamine hydrochloride; sodium hydroxide In ethanol; water at 20℃; | 73% |

| Conditions | Yield |
|---|---|
| 94% | |
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate 2: borneol dehydrogenase 2 from Salvia officinalis L.; NAD / acetonitrile; water / 24 h / 20 °C / pH 8 / Resolution of racemate; Enzymatic reaction View Scheme |
-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In chloroform; dimethyl sulfoxide at 25℃; for 240h; Mechanism; Darkness; | 94% |
-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
76-22-2
Camphor

-
-
61136-72-9, 63028-06-8, 140147-15-5
1-camphenyl triflate

| Conditions | Yield |
|---|---|
| With MDTBP In dichloromethane for 48h; Ambient temperature; | 93% |

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; magnesium In tetrahydrofuran at 10 - 25℃; for 1h; | 92% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene at 130℃; for 12h; Dean-Stark; Inert atmosphere; | 92% |
-
-
76-22-2
Camphor

-
-
1126-91-6, 28973-92-4, 74867-81-5, 111056-61-2
1,8,8-trimethyl-2-oxabicyclo(3.2.1)oct-3-one

-
-
507-96-0, 30860-35-6
1,8,8-trimethyl-3-oxabicyclo(3.2.1)oct-2-one

| Conditions | Yield |
|---|---|
| With bis-trimethylsilanyl peroxide; 1-(n-butyl)-3-methylimidazolium triflate at 40℃; for 15h; Baeyer-Villiger oxidation; Ionic liquid; Inert atmosphere; | A 89% B n/a |
| With 3-chloro-benzenecarboperoxoic acid Yield given. Yields of byproduct given. Title compound not separated from byproducts; |
-
-
76-22-2
Camphor

-
-
1010-45-3, 34733-73-8, 123355-20-4
<3,3-(2)H2>camphor

| Conditions | Yield |
|---|---|
| With water-d2; sodium In 1,4-dioxane at 50℃; for 168h; | 88% |

| Conditions | Yield |
|---|---|
| With sodium hydride In toluene at 60℃; for 1h; | 88% |
-
-
76-22-2
Camphor

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20 - 23℃; | 81% |
-
-
76-22-2
Camphor

-
-
536-74-3
phenylacetylene

-
-
124620-27-5
(+/-)-2-endo-(2-phenyl-1-ethynyl)-2-exo-hydroxy-1,7,7-trimethylbicyclo[2.2.1]heptane

| Conditions | Yield |
|---|---|
| Stage #1: phenylacetylene With n-butyllithium In tetrahydrofuran; hexane at -40 - -20℃; for 1h; Stage #2: Camphor In tetrahydrofuran; hexane at -20 - 20℃; Stage #3: With water In tetrahydrofuran; hexane | 80% |

| Conditions | Yield |
|---|---|
| at 100℃; for 5h; Inert atmosphere; | A n/a B 80% C n/a |


| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene at 110℃; | 78% |

| Conditions | Yield |
|---|---|
| With trimethyl orthoformate at 20℃; for 24h; | 77% |

| Conditions | Yield |
|---|---|
| Stage #1: phenylacetylene With n-butyllithium In tetrahydrofuran; hexane at -40 - -20℃; for 1h; Stage #2: Camphor In tetrahydrofuran; hexane at -20 - 20℃; Stage #3: ethyl iodide In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide; hexane at 20℃; for 18h; | 75% |

Camphor History
In the 9th century, the Arab chemist, Al-Kindi, provided the earliest recipe for the production of camphor in his Kitab Kimiya' al-'Itr.
Already in the 19th century, it was known that with nitric acid, camphor could be oxidized into camphoric acid. Haller and Blanc published a semisynthesis of camphor from camphoric acid, which, although demonstrating its structure, would not prove it. In 1903,the first complete total synthesis for camphoric acid was published by Gustaf Komppa.
In 1907, Komppa began industrial production of camphor in Tainionkoski, Finland.
Camphor Standards and Recommendations
OSHA PEL: TWA 2 mg/m3
ACGIH TLV: TWA 2 ppm; STEL 3 ppm; Not Classifiable as a Human Carcinogen
DFG MAK: 2 ppm (13 mg/m3)
DOT Classification: 4.2; Label: Flammable Solid
Camphor Analytical Methods
For occupational chemical analysis use NIOSH: Ketones II (desorption in 99:1 CS2:methanol) 1301.
Camphor Specification
The Camphor with CAS registry number of 76-22-2 is also known as Norcamphor, 1,7,7-trimethyl-. The IUPAC name is 4,7,7-Trimethylbicyclo[2.2.1]heptan-3-one. It belongs to product categories of Fine Chemical & Intermediates; Miscellaneous Natural Products; Ketone; Bicyclic Monoterpenes; Biochemistry; Camphor, etc. (Plasticizer); Functional Materials; Plasticizer; Terpenes. Its EINECS registry number is 200-945-0. In addition, the formula is C10H16O and the molecular weight is 152.23. This chemical is a colourless solid and should be stored in cool and dry place.
Physical properties about Camphor are: (1)ACD/LogP: 2.13; (2)ACD/LogD (pH 5.5): 2.13; (3)ACD/LogD (pH 7.4): 2.13; (4)ACD/BCF (pH 5.5): 24.39; (5)ACD/BCF (pH 7.4): 24.39; (6)ACD/KOC (pH 5.5): 342.47; (7)ACD/KOC (pH 7.4): 342.47; (8)#H bond acceptors: 1; (9)Index of Refraction: 1.485; (10)Molar Refractivity: 44.39 cm3; (11)Molar Volume: 154.8 cm3; (12)Surface Tension: 31.5 dyne/cm; (13)Density: 0.982 g/cm3; (14)Flash Point: 64.4 °C; (15)Enthalpy of Vaporization: 44.37 kJ/mol; (16)Boiling Point: 207.4 °C at 760 mmHg; (17)Vapour Pressure: 0.225 mmHg at 25 °C.
Preparation of Camphor: it is prepared by reaction of Turpentine. Camphene is obtained by the isomerization after pinene extraction. Then product is obtained by esterification, hydrolysis and dehydrogenation of camphen.
Uses of Camphor: it is used as chemical-based raw material and pharmaceutical intermediate. It is used to produce camphor-trimethylsilyl-enolether by reaction with chloro-trimethyl-silane. The reaction occurs with reagents NaI, triethylamine and solvents acetonitrile, pentane at ambient temperature for 96 hoursfor hours. The yield is about 59%.
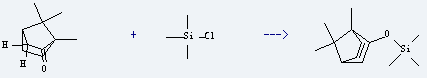
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. It is harmful by inhalation, in contact with skin and if swallowed. What's more, it is highly flammable. During using it, wear suitable protective clothing and eye/face protection. Keep away from sources of ignition. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CC1(C2CCC1(C(=O)C2)C)C
2. InChI: InChI=1S/C10H16O/c1-9(2)7-4-5-10(9,3)8(11)6-7/h7H,4-6H2,1-3H3
3. InChIKey: DSSYKIVIOFKYAU-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | intraperitoneal | 400mg/kg (400mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| child | LDLo | unreported | 100mg/kg (100mg/kg) | Yakkyoku. Pharmacy. Vol. 31, Pg. 1499, 1980. | |
| child | TDLo | oral | 51mg/kg (51mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | American Journal of Emergency Medicine. Vol. 7, Pg. 41, 1989. |
| dog | LDLo | oral | 800mg/kg (800mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1289, 1935. | |
| frog | LDLo | subcutaneous | 240mg/kg (240mg/kg) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 50, Pg. 199, 1903. |
| infant | LDLo | oral | 70mg/kg (70mg/kg) | SENSE ORGANS AND SPECIAL SENSES: MYDRIASIS (PUPILLARY DILATION): EYE BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | American Journal of Pathology. Vol. 30, Pg. 857, 1954. |
| man | LDLo | unreported | 29mg/kg (29mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| mouse | LCLo | inhalation | 400mg/m3/3H (400mg/m3) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 22(11), Pg. 83, 1957. |
| mouse | LD50 | intraperitoneal | 3gm/kg (3000mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: REGIDITY BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | American Journal of Pathology. Vol. 30, Pg. 857, 1954. |
| mouse | LD50 | oral | 1310mg/kg (1310mg/kg) | Shika Gakuho. Journal of Dentistry. Vol. 75, Pg. 934, 1975. | |
| mouse | LDLo | subcutaneous | 200mg/kg (200mg/kg) | BEHAVIORAL: EXCITEMENT | "Pharmakologische Prufung von Analgetika, Dissertation," Herrlen, G., Pharmakologischen Institut der Universitat Tubingen, Fed. Rep. Ger., 1933Vol. -, Pg. -, 1933. |
| rabbit | LDLo | oral | 2gm/kg (2000mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: REGIDITY | American Journal of Pathology. Vol. 30, Pg. 857, 1954. |
| rat | LD50 | subcutaneous | 70mg/kg (70mg/kg) | "Ueber die Pharmakologische Wirkung eines dem Pentamethylentetrazol Vol. -, Pg. -, 1934. | |
| rat | LDLo | intraperitoneal | 900mg/kg (900mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Journal of Pharmacology and Experimental Therapeutics. Vol. 65, Pg. 275, 1939. |
Related Products
- Camphor
- Camphorated oil
- Camphorated phenol
- 7622-23-3
- 7622-29-9
- 762239-61-2
- 762240-09-5
- 762240-92-6
- 762260-71-9
- 762262-07-7
- 762262-09-9
- 762262-11-3
- 762274-49-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View