 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
-
Name
CANNABIDIOL
- EINECS 200-659-6
- CAS No. 13956-29-1
- Article Data50
- CAS DataBase
- Density 1.025 g/cm3
- Solubility
- Melting Point 62-63°C
- Formula C21H30 O2
- Boiling Point 463.9 °C at 760 mmHg
- Molecular Weight 314.468
- Flash Point 206.3 °C
- Transport Information UN 1230 3
- Appearance
- Safety Poison by intravenous route. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and fumes.
- Risk Codes 11-23/24/25-39/23/24/25
-
Molecular Structure
-
Hazard Symbols
 F
F T
T
- Synonyms 1,3-Benzenediol,2-[3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-, (1R-trans)-;Cannabidiol (7CI); Resorcinol, 2-p-mentha-1,8-dien-3-yl-5-pentyl-, trans-(-)-(8CI); (-)-CBD; (-)-Cannabidiol; (-)-trans-Cannabidiol; CBD; D1(2)-trans-Cannabidiol
- PSA 40.46000
- LogP 5.84650
Synthetic route

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| With methylmagnesium bromide In diethyl ether; toluene at 110℃; for 12h; Solvent; Reagent/catalyst; Temperature; Inert atmosphere; | 99% |
| With potassium hydroxide In methanol; dichloromethane at 20℃; Inert atmosphere; | 91% |
-
-
1242-67-7
O-2797

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at 0℃; for 2h; Solvent; | 97% |
| With methyl magnesium iodide In diethyl ether at 0 - 160℃; for 1.5h; Inert atmosphere; | 62% |
| With sodium thioethylate In N,N-dimethyl-formamide at 150℃; for 5h; Temperature; Reagent/catalyst; Solvent; Inert atmosphere; | 1.02 g |

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 90℃; for 4h; Inert atmosphere; | 94% |

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-4-methyl-phenol; lithium chloride In dimethyl sulfoxide at 80 - 100℃; Reagent/catalyst; Solvent; Temperature; | 93% |

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 95℃; for 8h; Inert atmosphere; | 92% |

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 95℃; for 8h; Inert atmosphere; | 91% |

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 95℃; for 8h; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: n-pentylmagnesium bromide With lithium chloride; zinc(II) chloride In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Stage #2: (1′R,2′R)-2,6-bis((tert-butoxycarbonyl)oxy)-5′-methyl-2′-(prop-1-en-2-yl)-1′,2′,3′,4′-tetrahydro-[1,1′-biphenyl]-4-yl trifluoromethanesulfonate With [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) In tetrahydrofuran at 20 - 60℃; | 86% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; tetrakis(triphenylphosphine) palladium(0) In toluene at 100℃; for 16h; Temperature; Suzuki Coupling; Inert atmosphere; | 80.7% |
-
-
52154-82-2
(1R,4R)-p-mentha-2,8-dien-1-ol

-
-
78463-34-0
4,6-dibromo-5-pentylbenzene-1,3-diol

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| Stage #1: (1R,4R)-p-mentha-2,8-dien-1-ol; 4,6-dibromo-5-pentylbenzene-1,3-diol With magnesium sulfate; toluene-4-sulfonic acid In dichloromethane at -20 - -15℃; Inert atmosphere; Stage #2: With triethylamine; sodium sulfite In methanol; water for 20h; Reflux; | 79% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium carbonate In toluene at 90℃; for 17h; Suzuki Coupling; Inert atmosphere; | 78.3% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; tetrakis(triphenylphosphine) palladium(0) In toluene at 100℃; for 16h; Suzuki Coupling; Inert atmosphere; | 77.6% |

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In methanol at 120℃; for 5h; Sealed tube; | 60% |
-
-
22972-51-6
(1S,4R)-p-mentha-2,8-dien-1-ol

-
-
500-66-3
Olivetol

-
A
-
13956-29-1
CBD

-
B
-
22972-55-0
abnormal cannabidiol

-
C
-
22972-53-8
(-)-2,4-Bis-<3,4-trans-p-menthadien-(1,8)-yl-(3)>-olivetol

| Conditions | Yield |
|---|---|
| With aluminum oxide; boron trifluoride diethyl etherate In dichloromethane at 40 - 41℃; for 0.00277778h; | A 55% B 14% C 6% |


-
-
500-66-3
Olivetol

-
A
-
13956-29-1
CBD

-
B
-
22972-55-0
abnormal cannabidiol

-
C
-
22972-53-8
(-)-2,4-Bis-<3,4-trans-p-menthadien-(1,8)-yl-(3)>-olivetol

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane for 0.116667h; Flow reactor; | A 55% B 19% C 4% |


| Conditions | Yield |
|---|---|
| MoCl2(acetylacetonate)2; silver trifluoromethanesulfonate In dichloromethane at -20℃; for 3h; | A 20% B 52% |


| Conditions | Yield |
|---|---|
| With zinc(II) chloride In dichloromethane; water at 40℃; for 1.5h; | 51.8% |

| Conditions | Yield |
|---|---|
| Stage #1: Olivetol With boron trifluoride diethyl etherate In dichloromethane at 0℃; for 0.333333h; Stage #2: (4R)-1-methyl-4-(2-(1-propylene))-2-cyclohexene-2-ol In dichloromethane at 25℃; | 48% |
| Stage #1: (4R)-1-methyl-4-(2-(1-propylene))-2-cyclohexene-2-ol In dichloromethane for 1h; Stage #2: Olivetol With zinc(II) chloride In dichloromethane; water at 0 - 40℃; for 0.833333h; |

| Conditions | Yield |
|---|---|
| Stage #1: Olivetol With zinc(II) chloride In dichloromethane at 40℃; for 1h; Stage #2: (+)-p-mentha-2,8-dien-1-ol In dichloromethane at 40℃; for 1.66667h; Stage #3: With boron trifluoride diethyl etherate In dichloromethane at -10℃; for 2.5h; Product distribution / selectivity; | A 0.71% B 5.06% C 45.1% |

-
-
140633-48-3
Phosphoric acid (5R,6R)-6-(2,6-dimethoxy-4-pentyl-phenyl)-5-isopropenyl-2-methyl-cyclohex-1-enyl ester diethyl ester

-
A
-
13956-29-1
CBD

-
B
-
1972-05-0
Cannabidiol monomethyl ether

| Conditions | Yield |
|---|---|
| With lithium; methylamine In tetrahydrofuran; tert-butyl alcohol at -10℃; for 1h; | A 35% B 43% |

| Conditions | Yield |
|---|---|
| Stage #1: n-pentylmagnesium bromide With lithium chloride; zinc(II) chloride In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: (1′R,2′R)-2,6-dihydroxy-5′-methyl-2′-(prop-1-en-2-yl)-1′,2′,3′,4′-tetrahydro-[1,1′-biphenyl]-4-yl trifluoromethanesulfonate With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium diacetate In tetrahydrofuran at 20 - 30℃; Inert atmosphere; | 30% |
| With potassium phosphate; [1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride In tetrahydrofuran; diethyl ether at 20℃; for 20h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: Olivetol With toluene-4-sulfonic acid In benzene for 2.5h; Stage #2: (1S,4R)-p-mentha-2,8-dien-1-ol In benzene at 20℃; for 0.5h; | 24% |
| With toluene-4-sulfonic acid In toluene at 18 - 25℃; for 1.5h; Inert atmosphere; | 20% |
| With oxalic acid |

| Conditions | Yield |
|---|---|
| With N,N-dimethylformamide dineopentyl acetal |
-
-
1686-20-0, 23727-14-2, 38344-42-2, 120523-29-7
p‐mentha‐1,5‐dien‐8‐ol

-
-
500-66-3
Olivetol

-
A
-
13956-29-1
CBD

-
B
-
5957-75-5
delta-8-tetrahydrocannabinol

-
C
-
1972-08-3
dronabinol

| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid In benzene at 25℃; for 1.58333h; Product distribution; |

-
-
20053-58-1
(1S,2S,3R,6R)-(+)-trans-car-2-ene epoxide

-
-
500-66-3
Olivetol

-
A
-
13956-29-1
CBD

-
B
-
22972-55-0
abnormal cannabidiol

-
C
-
5957-75-5
delta-8-tetrahydrocannabinol

-
D
-
1972-08-3
dronabinol

| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid In benzene at 40℃; for 0.75h; Product distribution; Mechanism; various temperatures; | A 24 % Chromat. B 32 % Chromat. C 6 % Chromat. D 38 % Chromat. |

-
-
936-91-4
3-carene epoxide

-
-
500-66-3
Olivetol

-
A
-
13956-29-1
CBD

-
B
-
22972-55-0
abnormal cannabidiol

-
C
-
5957-75-5
delta-8-tetrahydrocannabinol

-
D
-
1972-08-3
dronabinol

| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid In benzene at 60℃; for 0.5h; Mechanism; Product distribution; |

-
-
40525-15-3
(1′R,2′R)-5′-methyl-4-pentyl-2′-(prop-1-en-2-yl)-1′,2′,3′,4′-tetrahydro-[1,1′-biphenyl]-2,6-diyl diacetate

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; tert-butyl alcohol Irradiation; |
-
-
20053-58-1
(1S,2S,3R,6R)-(+)-trans-car-2-ene epoxide

-
-
500-66-3
Olivetol

-
A
-
13956-29-1
CBD

-
B
-
5957-75-5
delta-8-tetrahydrocannabinol

-
C
-
1972-08-3
dronabinol

| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid In benzene at 20℃; Product distribution; Mechanism; investigation with (+)-p-menthadienol; |

-
-
10293-10-4
3-endo-9-dibromo-1,7,7-trimethylbicyclo<2.2.1>heptan-2-one

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1) CuI / 1) THF, 0 deg C, 20 min, 2) DMSO, THF, r.t., overnight 2: 1) sodium naphthalenide, 2) HMPA / 1) THF, tetraethyleneglycol dimethyl ether, -78 deg C, 2) -20 deg C 3: 35 percent / Li, MeNH2 / tetrahydrofuran; 2-methyl-propan-2-ol / 1 h / -10 °C View Scheme |
-
-
13956-29-1
CBD

-
-
108-24-7
acetic anhydride

-
-
40525-15-3
(1′R,2′R)-5′-methyl-4-pentyl-2′-(prop-1-en-2-yl)-1′,2′,3′,4′-tetrahydro-[1,1′-biphenyl]-2,6-diyl diacetate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane for 4h; | 100% |
| With pyridine at 20℃; for 48h; Inert atmosphere; | 66% |
| With pyridine; dmap at 20℃; for 16h; | 44% |

| Conditions | Yield |
|---|---|
| for 0.75h; | 100% |
| In n-heptane at 20℃; for 15h; Solvent; Temperature; Inert atmosphere; | 50% |
-
-
13956-29-1
CBD

-
-
1972-08-3
dronabinol

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane at -10 - 0℃; for 3h; Product distribution / selectivity; | 99% |
| With boron trifluoride diethyl etherate In dichloromethane at -10 - 20℃; for 1h; | 83% |
| With boron trifluoride diethyl etherate In dichloromethane at -10 - 0℃; for 3h; Solvent; Temperature; Inert atmosphere; | 71.9% |
-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| Stage #1: CBD With potassium hydroxide In ethanol at 20℃; for 16h; Inert atmosphere; Stage #2: With hydrogenchloride In water | 99% |
-
-
13956-29-1
CBD

-
-
96-32-2
bromoacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 4h; Methylation; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 24h; Inert atmosphere; | 78% |
| With potassium carbonate; acetone |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 4h; | 98% |
-
-
13956-29-1
CBD

-
-
877660-90-7
8,9-dihydro-cannabidiol

| Conditions | Yield |
|---|---|
| With hydrogen; platinum In ethyl acetate under 517.162 Torr; for 0.5h; | 97.5% |
| With platinum(IV) oxide; hydrogen In ethyl acetate at 20℃; under 517.162 Torr; for 0.0333333h; | 97.5% |
| With platinum(IV) oxide; hydrogen In ethyl acetate under 517.162 Torr; for 0.0333333h; | 97.5% |
-
-
13956-29-1
CBD

-
-
123421-01-2
2-(6-methyl-3-(prop-1-en-2-yl)-7-oxabicyclo[4.1.0]heptan-2-yl)-5-pentylbenzene-1,3-diol

| Conditions | Yield |
|---|---|
| With Oxone In acetone at 20℃; | 95% |
| With dihydrogen peroxide; potassium hydrogencarbonate; benzonitrile In methanol at 20℃; for 40h; Inert atmosphere; | 43% |
-
-
13956-29-1
CBD

-
-
100-51-6
benzyl alcohol

| Conditions | Yield |
|---|---|
| Stage #1: CBD; benzyl alcohol With triphenylphosphine In tetrahydrofuran at 0℃; for 0.5h; Mitsunobu Displacement; Inert atmosphere; Stage #2: With di-isopropyl azodicarboxylate In tetrahydrofuran at 20℃; Mitsunobu Displacement; Inert atmosphere; | 94% |
-
-
13956-29-1
CBD

-
-
100-39-0
benzyl bromide

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With Amberlyst-15 In n-heptane for 1h; Reagent/catalyst; Solvent; Reflux; | A 85% B n/a |
| With aluminum isopropoxide at 90 - 180℃; for 3h; Large scale; | A 85% B n/a |
| With Amberlyst-15 In n-heptane at 60℃; for 2h; Reagent/catalyst; Temperature; | A 81.1% B 5.3% |
-
-
13956-29-1
CBD

-
-
106-95-6
allyl bromide

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; Inert atmosphere; | 84% |

| Conditions | Yield |
|---|---|
| In n-heptane at 20℃; for 15h; Solvent; Temperature; | 80% |

| Conditions | Yield |
|---|---|
| In n-heptane at 20℃; for 15h; Solvent; Temperature; | 80% |
-
-
13956-29-1
CBD

-
-
137252-25-6
HU-331

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In toluene at 20℃; for 4h; | 74% |
| With potassium tert-butylate In toluene at 20℃; for 4h; Reagent/catalyst; Solvent; | 74% |
| With stabilized 1-hydroxy-1λ5,2-benziodoxole-1,3-dione, SIBX In ethyl acetate at 20℃; for 18h; Cooling with ice; | 61% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| With platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex In toluene at 20 - 30℃; for 2.5h; | 74% |
-
-
1873-88-7
1,1,1,3,5,5,5-heptamethyltrisiloxan

-
-
13956-29-1
CBD

| Conditions | Yield |
|---|---|
| With platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex In 5,5-dimethyl-1,3-cyclohexadiene; toluene at 20 - 30℃; for 2.5h; | 74% |
Cannabidiol Chemical Properties
Chemistry informtion about Cannabidiol (CAS NO.13956-29-1) is:
IUPAC Name: 2-[(6r)-3-Methyl-6-Prop-1-En-2-Ylcyclohex-2-En-1-Yl]-5-Pentylbenzene-1,3-Diol
Synonyms: (-)-Trans-2-P-Mentha-1,8-Dien-3-Yl-5-Pentylresorcinol ; (-)-Trans-Cannabidiol ; ,(1r-Trans)- ; 1,3-Benzenediol, 2-[3-Methyl-6-(1-Methylethenyl)-3-Cyclohexen-1-Yl]-5-Pentyl- ; 2-(3-Methyl-6-(1-Methylethenyl)-2-Cyclohexen-1-Yl)-5-Pentyl-3-Benzenediol ; 2-(6-Isopropenyl-3-Methyl-2-Cyclohexen-1-Yl)-5-Pentyl-1,3-Benzenediol ; 8-Dien-3-Yl-5-Pentyl-2-P-Mentha-(-)-(E)-Resorcino ; Delta1(2)-Trans-Cannabidiol
Product Categories: Cannabinoid receptor
MF: C21H30O2
MW: 314.46
Density: 1.025 g/cm3
Flash Point: 206.3 °C
Boiling Point: 463.9 °C at 760 mmHg
Vapour Pressure: 3.14E-09 mmHg at 25°C
Enthalpy of Vaporization: 75.31 kJ/mol
Storage temp.: 2-8°C
Following is the molecular structure of Cannabidiol (CAS NO.13956-29-1) is:
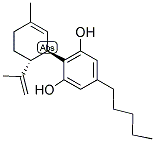
Cannabidiol Uses
In April 2005, Canadian authorities approved the marketing of Sativex, a mouth spray for multiple sclerosis to alleviate pain. Sativex contains tetrahydrocannabinol together with Cannabidiol (CAS NO.13956-29-1). Initial research is showing that CBD has an effect in reducing schizophrenic symptoms in patients. Further research has verified these results. Leweke et al., (2009) performed a double blind, 4 week, explorative study controlled clinical trial, to compare the effects of purified cannabidiol and the atypical antipsychotic amisulpride on improving the symptoms of schizophrenia in 42 patients with acute schizophrenia. 'Both treatments were associated with a significant decrease of psychotic symptoms after 2 and 4 weeks as assessed by BPRS and PANSS. However, there was no statistical difference between both treatment groups. In contrast, cannabidiol induced significantly less side effects (EPS, increase in prolactin, weight gain) when compared to amisulpride'. The authors conclude cannabidiol revealed substantial antipsychotic properties in acute paranoid schizophirenia (Leweke et al., 2009). This led the authors to suggest the endocannabinoid system plays an adaptive role in the development of paranoid schizophirenia and that this research provides evidence that this mechanism may be a valuable target for 'antipsychotic treament strategies' .
Cannabidiol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | intravenous | > 254mg/kg (254mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 88, Pg. 154, 1946. | |
| monkey | LD50 | intravenous | 212mg/kg (212mg/kg) | behavioral: muscle contraction or spasticity) cardiac: arrhythmias (including changes in conduction) lungs, thorax, or respiration: dyspnea | Toxicology and Applied Pharmacology. Vol. 58, Pg. 118, 1981. |
| mouse | LD50 | intravenous | 50mg/kg (50mg/kg) | Journal of Medicinal Chemistry. Vol. 18, Pg. 213, 1975. |
Cannabidiol Consensus Reports
EPA Genetic Toxicology Program.
Cannabidiol Safety Profile
Poison by intravenous route. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and fumes.
Hazard Codes:
 F
F
 T
T
Risk Statements:
R11:Highly flammable.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
R39/23/24/25:Toxic by inhalation, in contact with skin and if swallowed and danger of very serious irreversible effects.
Safety Statements:
S36/37:Wear suitable protective clothing and gloves.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S16:Keep away from sources of ignition.
S7:Keep container tightly closed.
RIDADR: UN 1230 3/PG 2
WGK Germany: 1
RTECS: VH1600000
Cannabidiol Specification
Cannabidiol (CAS NO.13956-29-1) is a psychoactive cannabinoid found in Cannabis. It is a major constituent of the plant, representing up to 40% in its extracts.It is not intoxicating but displayed sedative effects in animal tests. Some research, however, indicates that CBD can increase alertness. It may decrease the rate of THC clearance from the body, perhaps by interfering with the metabolism of THC in the liver. CBD appears to affect both the CB1 and CB2 receptors- with higher affinity for the CB2 receptors . Cannabis indica dominant strains of the plant are known to be higher in CBD than Cannabis sativa strains. Medically, it appears to relieve convulsion, inflammation, anxiety, and nausea, and to inhibit cancer cell growth. Recent studies have shown cannabidiol to be as effective as atypical antipsychotics in treating schizophrenia.
Related Products
- Cannabidiol
- Cannabidiol hydroxyquinone
- 13956-51-9
- 13956-52-0
- 139569-17-8
- 13957-31-8
- 13958-16-2
- 139585-48-1
- 139585-70-9
- 13958-86-6
- 13958-88-8
- 13958-91-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View