-
Name
Carbobenzoxyhydrazide
- EINECS 226-230-3
- CAS No. 5331-43-1
- Article Data15
- CAS DataBase
- Density 1.202 g/cm3
- Solubility Soluble in water (slightly), methanol and DMSO.
- Melting Point 65-68 °C(lit.)
- Formula C8H10N2O2
- Boiling Point 351.292 °C at 760 mmHg
- Molecular Weight 166.18
- Flash Point 166.255 °C
- Transport Information
- Appearance light beige shiny flakes
- Safety 26-36
- Risk Codes 41
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Carbazicacid, benzyl ester (6CI,7CI,8CI);Benzylhydrazinecarboxylate;Benzyl hydrazinocarboxylate;Benzyloxycarbonyl hydrazide;Hydrazincarboxylic acid benzylester;NSC 2287;[(Benzyloxy)carbonyl]hydrazine;Carbazic acid, benzyl ester;
- PSA 64.35000
- LogP 1.87770
Synthetic route
-
-
202980-89-0
C11H11Cl3N2O4

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; mischmetal (50 percent Ce, 25 percent La, 16 percent Nd, 6 percent Pr) In tetrahydrofuran for 3h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With lithium carbonate; hydrazine hydrate In ethyl acetate at 30 - 120℃; for 2.75h; Temperature; Solvent; Reagent/catalyst; Inert atmosphere; | 95% |
| With potassium carbonate; hydrazine hydrate In tetrahydrofuran at -20℃; for 2h; | 90% |
| With hydrazine hydrate In diethyl ether for 1h; Acylation; | 82% |
| With chloroform; hydrazine hydrate | |
| (i) N2H4*H2O, (ii) HCl; Multistep reaction; |
-
-
22129-07-3
N-benzyloxycarbonylimidazole

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In tetrahydrofuran at 20℃; | 95% |
| With hydrazine hydrate In dichloromethane at 0 - 20℃; for 1.5h; | 5.78 g |

| Conditions | Yield |
|---|---|
| In diethyl ether at -5 - 20℃; for 2h; | 58% |
-
-
6294-89-9
hydrazinecarboxylic acid methyl ester

-
-
100-51-6
benzyl alcohol

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 39% |
-
-
13795-24-9
benzyl 4-nitrophenyl carbonate

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In dichloromethane at 0 - 20℃; Inert atmosphere; | 24% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate at 110℃; | |
| With hydrazine hydrate auf dem Dampfbad; | |
| With hydrazine hydrate In isopropyl alcohol |

-
A
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) Na, petroleum ether, (ii) /BRN= 471308/, KI, DMF 2: N2H4*H2O / propan-2-ol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dichloromethane / 1 h / 20 °C 2: hydrazine hydrate / dichloromethane / 1.5 h / 0 - 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; | 77% |
-
-
127406-56-8
2-(4-formylphenyl)pyridine

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
1003888-34-3
C20H17N3O2

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 80℃; for 16h; | 100% |
-
-
1093214-91-5
1-(tert-butoxycarbonyl)-3-(ethoxycarbonyl)piperidine-3-carboxylic acid

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
1186019-61-3
1-tert-butyl 3-ethyl 3-({2-[(benzyloxy)carbonyl]hydrazino}carbonyl)piperidine-1,3-dicarboxylate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 16h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethyl acetate at 50℃; for 16h; Reagent/catalyst; | 100% |
-
-
175211-73-1
methyl N-{[(1,1-dimethylethyl)oxy]carbonyl}-N-(2-oxoethyl)glycinate

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

| Conditions | Yield |
|---|---|
| With sodium sulfate In methanol at 20℃; for 2h; | 100% |
-
-
55820-40-1, 34710-33-3, 97859-03-5
Pd(t-BuNC)2Cl2

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 5h; | 100% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
57699-88-4
1-tert-butoxycarbonyl-2-carbobenzyloxyhydrazine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 99% |
| With dmap; triethylamine In dichloromethane for 24h; | 90% |
| With triethylamine In tetrahydrofuran at 20℃; for 16h; Inert atmosphere; | 89% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
67-64-1
acetone

-
-
3057-26-9
2-(propan-2-yliden)hydrazinecarboxylic acid benzyl ester

| Conditions | Yield |
|---|---|
| In toluene at 20 - 60℃; for 26h; | 99% |
| With triethylamine In methanol for 2h; Heating; | 97% |
| In toluene a.) 50 degC, 30 min, b.) room temp., 12 h; | 94% |

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
1003888-35-4
C22H23N3O2S

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 80℃; for 18h; | 99% |
-
-
624-31-7
4-tolyl iodide

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
1197055-07-4
benzyl 1-p-tolylhydrazinecarboxylate

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 1,10-Phenanthroline; caesium carbonate In N,N-dimethyl-formamide at 20 - 80℃; Inert atmosphere; | 99% |
-
-
35779-04-5
1-tert-butyl-4-iodobenzene

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
1197055-09-6
benzyl 1-(4-tert-butylphenyl)hydrazinecarboxylate

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 1,10-Phenanthroline; caesium carbonate In N,N-dimethyl-formamide at 20 - 80℃; Inert atmosphere; | 99% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
14763-00-9, 14865-30-6
4,5-diphenylocta-1,7-diyne-4,5-diol

-
-
1252032-33-9
benzyl (2,5-dimethyl-3a,6a-diphenylhexahydro-2,5-epiminofuro[3,2-b]furan-7-yl)carbamate

| Conditions | Yield |
|---|---|
| With (N'-(tbutyl)-N,N-diethylcarbamimidoyl)gold(I) chloride; silver(I) triflimide In acetonitrile at 20℃; for 16h; Inert atmosphere; | 99% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
78-84-2
isobutyraldehyde

-
-
1451982-81-2
(2E)-(2-methylpropyliden)hydrazinecarboxylic acid benzyl ester

| Conditions | Yield |
|---|---|
| In toluene at 20 - 60℃; for 26h; | 99% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
3934-87-0
5-hydroxyvanillin

-
-
1621252-64-9
(E)-benzyl 2-(3,4-dihydroxy-5-methoxybenzylidene)hydrazinecarboxylate

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 99% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
121-33-5
vanillin

-
-
1621252-65-0
(E)-benzyl 2-(4-hydroxy-3-methoxybenzylidene)hydrazinecarboxylate

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 99% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
139-85-5
3,4-dihydroxybenzaldehyde

-
-
1621252-66-1
(E)-benzyl 2-(3,4-dihydroxybenzylidene)hydrazinecarboxylate

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 99% |
-
-
51061-83-7
2,4-dihydroxy-5-methoxybenzaldehyde

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
1621252-68-3
(E)-benzyl 2-(2,4-Dihydroxy-5-methoxybenzylidene)hydrazinecarboxylate

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 99% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 98% |
| In hexane for 8h; Heating; | 79% |
| In methanol at 65℃; |

| Conditions | Yield |
|---|---|
| In hexane for 8h; Heating; | 98% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
97297-40-0
N'-((DL)-2-tert-butoxycarbonylamino-3-phenyl-propionyl)-hydrazine carboxylic acid benzyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-(tert-butoxycarbonylamino)-3-phenylpropionic acid With benzotriazol-1-ol; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 0.5h; Stage #2: N-benzyloxycarbonyl-hydrazine In dichloromethane at 20℃; | 98% |
-
-
766-85-8
3-methoxy-1-iodobenzene

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
1197055-14-3
benzyl 1-(3-methoxyphenyl)hydrazinecarboxylate

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 1,10-Phenanthroline; caesium carbonate In N,N-dimethyl-formamide at 20 - 80℃; Inert atmosphere; | 98% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
86-81-7
3,4,5-trimethoxy-benzaldehyde

-
-
1621252-63-8
(E)-benzyl 2-(3,4,5-trimethoxybenzylidene)hydrazinecarboxylate

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 98% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25 - 30℃; for 16h; | 98% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
61771-76-4
1-(but-3-enyl)-2-oxocyclohexanecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In ethanol | 98% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

| Conditions | Yield |
|---|---|
| In ethanol | 98% |

| Conditions | Yield |
|---|---|
| In ethanol; water at 80℃; for 4h; | 98% |
-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
90-02-8
salicylaldehyde

-
-
1621252-67-2
benzyl (E)-2-(2-hydroxybenzylidene)hydrazine-1-carboxylate

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 0.666667h; | 97.8% |
| In ethanol Reflux; | 91.5% |
-
-
696-62-8
para-iodoanisole

-
-
5331-43-1
N-benzyloxycarbonyl-hydrazine

-
-
40093-52-5
benzyl 1-(4-methoxyphenyl)hydrazinecarboxylate

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 1,10-Phenanthroline; caesium carbonate In N,N-dimethyl-formamide at 20 - 80℃; Inert atmosphere; | 97% |
| With copper(l) iodide; 1,10-Phenanthroline; caesium carbonate In N,N-dimethyl-formamide at 80℃; for 1h; Inert atmosphere; | 51% |

| Conditions | Yield |
|---|---|
| at 15℃; for 16h; | 97% |
Carbobenzoxyhydrazide Specification
The Carbobenzoxyhydrazide, with the CAS registry number 5331-43-1, is also known as Carbazic acid, benzyl ester. It belongs to the product category of Phosgene Derivatives. Its EINECS number is 226-230-3. This chemical's molecular formula is C8H10N2O2 and molecular weight is 166.18. What's more, its systematic name is Benzyl hydrazinecarboxylate. This chemical is stable at common pressure and temperature, and it should be sealed and stored in a cool and dry place. Moreover, it should be protected from light. It is used to synthetize peptide.
Physical properties of Carbobenzoxyhydrazide are: (1)ACD/LogP: 0.777; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.78; (4)ACD/LogD (pH 7.4): 0.78; (5)ACD/BCF (pH 5.5): 2.29; (6)ACD/BCF (pH 7.4): 2.29; (7)ACD/KOC (pH 5.5): 62.81; (8)ACD/KOC (pH 7.4): 63.00; (9)#H bond acceptors: 4; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 64.35 Å2; (13)Index of Refraction: 1.56; (14)Molar Refractivity: 44.707 cm3; (15)Molar Volume: 138.226 cm3; (16)Polarizability: 17.723 10-24cm3; (17)Surface Tension: 48.9 dyne/cm; (18)Density: 1.202 g/cm3; (19)Flash Point: 166.255 °C; (20)Enthalpy of Vaporization: 59.598 kJ/mol; (21)Boiling Point: 351.292 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation: this chemical can be prepared by carbonochloridic acid benzyl ester. This reaction will need reagent hydrazine monohydrate and solvent diethyl ether with the reaction time of 1 hour. The yield is about 82%.

Uses of Carbobenzoxyhydrazide: it can be used to produce N-Methylenbenzylcarbazat by heating. It will need reagent triethylamine and solvent methanol with the reaction time of 2 hours. The yield is about 95%.
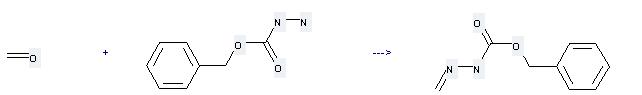
When you are using this chemical, please be cautious about it as the following:
This chemical has a risk of serious damage to eyes. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCc1ccccc1)NN
(2)Std. InChI: InChI=1S/C8H10N2O2/c9-10-8(11)12-6-7-4-2-1-3-5-7/h1-5H,6,9H2,(H,10,11)
(3)Std. InChIKey: RXUBZLMIGSAPEJ-UHFFFAOYSA-N
Related Products
- Carbobenzoxyhydrazide
- 5331-48-6
- 533-15-3
- 53316-51-1
- 533-17-5
- 5331-91-9
- 5331-92-0
- 53319-52-1
- 5332-06-9
- 53320-86-8
- 53321-09-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View