-
Name
Clofarabine
- EINECS 631-422-9
- CAS No. 123318-82-1
- Article Data20
- CAS DataBase
- Density 2.12 g/cm3
- Solubility
- Melting Point 228-231 °C
- Formula C10H11ClFN5O3
- Boiling Point 550 °C at 760 mmHg
- Molecular Weight 303.68
- Flash Point 286.4 °C
- Transport Information
- Appearance white solid
- Safety 45
- Risk Codes 25
-
Molecular Structure
- Hazard Symbols T
- Synonyms C1-F-Ara-A;9H-Purin-6-amine, 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)-;2-Chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine;Clofarabine, 5-(6-Amino-2-chloro-purin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol;5-(6-Amino-2-chloro-purin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol;Clofarex;Clolar;2-Chloro-9-(2-deoxy-2-fluoro-b-D-arabinofuranosyl)-9H-purin-6-amine;(2R,3R,4S,5R)-5-(6-amino-2-chloro-purin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol;CAFDA;9H-Purin-6-amine, 2-chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)-;Clofarabine(Clofarabine or called Keluola foreshore);2-Chloro-6-amino-purine-2'-fluoro-2'- deoxy arabineoside;
- PSA 119.31000
- LogP 0.23180
Synthetic route
-
-
355138-50-0
6-amino-2-chloro-9-(2'-deoxy-2'-fluoro-3',5'-di-O-benzoyl-β-D-arabinofuranosyl)-9H-purine

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| With methanol; sodium methylate at 15 - 20℃; for 2h; | 95.41% |
| With methanol; sodium methylate at 20℃; for 2h; pH=9 - 10; Reagent/catalyst; Green chemistry; | 95.5% |
| With methanol; sodium methylate at 0℃; for 2h; | 95.5% |
-
-
1234346-14-5
6-amino-2-chloro-9-(2-deoxy-2-fluoro-3,5-di-O-(triisopropylsilyl)-β-D-arabinofuranosyl)-9H-purine

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| With tetramethylammonium fluoride; acetic acid In N,N-dimethyl-formamide at 20℃; | 90% |
| With tetramethylammonium fluoride; acetic acid In N,N-dimethyl-formamide at 20℃; | 90% |

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 60℃; for 5h; | 90% |

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| 60% |

-
-
1839-18-5
2-chloroadenine

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| With recombinant E.coli purine nucleoside phosphorylase In water at 52℃; for 168h; Enzymatic reaction; | 42% |
-
-
103884-99-7
2,6-dichloro-9-(3-O-acetyl-5-O-benzoyl-2-deoxy-2-fluoro-β-D-arabinofuranosyl)-9H-purine

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| With lithium hydroxide; ammonia 1.) ethanol, RT, 3 d, 2.) acetonitrile, RT, 3 h; Yield given. Multistep reaction; |
-
-
1353040-42-2
2-chloro-9-(3',5'-di-O-benzoyl-β-D-ribofuranosyl)adenine

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: pyridine / dichloromethane / 0 - 20 °C / Inert atmosphere 2.1: guanidine carbonate / dichloromethane; ethyl acetate / 60 °C 2.2: 80 °C 3.1: sodium methylate; methanol / 30 - 40 °C View Scheme | |
| Multi-step reaction with 3 steps 1: pyridine; dichloromethane / 0 - 20 °C / Inert atmosphere 2: guanidine hydrogen carbonate; triethylamine hydrofluoride / ethyl acetate / 20 - 80 °C 3: sodium methylate / methanol / 30 - 40 °C View Scheme | |
| Multi-step reaction with 3 steps 1: pyridine / dichloromethane / 0.5 h / -10 - 0 °C / Inert atmosphere 2: N-ethyl-N,N-diisopropylamine; triethylamine tris(hydrogen fluoride) / toluene / 48 h / 35 - 40 °C 3: sodium methylate / methanol / 0.5 h / 30 °C View Scheme |
-
-
1055168-98-3
6-amino-2-chloro-9-(2',3',5'-tri-O-benzoyl-β-D-ribofuranosyl)-9H-purine

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: pyridine; hydrazine hydrate; acetic acid / 75 - 80 °C / Inert atmosphere 2.1: pyridine / dichloromethane / 0 - 20 °C / Inert atmosphere 3.1: guanidine carbonate / dichloromethane; ethyl acetate / 60 °C 3.2: 80 °C 4.1: sodium methylate; methanol / 30 - 40 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: pyridine; hydrazine hydrate; acetic acid / 75 - 80 °C / Inert atmosphere 1.2: 65 °C 2.1: pyridine / dichloromethane / 0 - 20 °C / Inert atmosphere 3.1: guanidine carbonate / dichloromethane; ethyl acetate / 60 °C 3.2: 80 °C 4.1: sodium methylate; methanol / 30 - 40 °C View Scheme | |
| Multi-step reaction with 4 steps 1: hydrazine / pyridine; acetic acid / 20 - 80 °C / Inert atmosphere 2: pyridine; dichloromethane / 0 - 20 °C / Inert atmosphere 3: guanidine hydrogen carbonate; triethylamine hydrofluoride / ethyl acetate / 20 - 80 °C 4: sodium methylate / methanol / 30 - 40 °C View Scheme |
-
-
1353040-43-3
2-chloro-9-(3',5'-di-O-benzoyl-2'-O-trifluoromethylsulfonyl-β-D-ribofuranosyl)adenine

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N-ethyl-N,N-diisopropylamine; triethylamine tris(hydrogen fluoride) / toluene / 48 h / 35 - 40 °C 2: sodium methylate / methanol / 0.5 h / 30 °C View Scheme |
-
-
97614-44-3
2-deoxy-2-fluoro-3,5-di-O-benzoyl-α-D-arabinofuranosyl bromide

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium iodide / acetonitrile; tert-Amyl alcohol / 24 h / 20 °C / Inert atmosphere 2: sodium methylate / methanol / 7 h / 33 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1.1: potassium tert-butylate / acetonitrile; 1,2-dichloro-ethane; tert-Amyl alcohol / 19 h / 50 °C / Inert atmosphere 2.1: sodium methylate / methanol / 5 h / 20 °C / Inert atmosphere 2.2: 20 - 64 °C View Scheme | |
| Multi-step reaction with 3 steps 1: calcium hydride; sodium hydride / dichloromethane; acetonitrile / 20 °C / Inert atmosphere 2: ammonia / acetonitrile / 20 °C 3: sodium methylate / methanol / 0.67 h / 30 - 35 °C View Scheme |

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / water / pH 7.5 2: recombinant E.coli purine nucleoside phosphorylase / water / 168 h / 52 °C / Enzymatic reaction View Scheme |
-
-
1839-18-5
2-chloroadenine

-
-
69123-94-0
1-(2'-deoxy-2'-fluoro-β-D-arabinofuranosyl)uracil

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloroadenine; 1-(2'-deoxy-2'-fluoro-β-D-arabinofuranosyl)uracil In aq. buffer at 50℃; for 0.5h; pH=6.5; Stage #2: With nucleoside deoxyribosyltransferase enzyme (NDT enzyme) In aq. buffer at 50℃; Concentration; Solvent; Temperature; Enzymatic reaction; |
-
-
146-77-0
2-Chloroadenosine

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: pyridine / acetonitrile / 0.5 h / -5 - 5 °C 1.2: Reflux 2.1: pyridine / dichloromethane / 0.5 h / -10 - 0 °C / Inert atmosphere 3.1: N-ethyl-N,N-diisopropylamine; triethylamine tris(hydrogen fluoride) / toluene / 48 h / 35 - 40 °C 4.1: sodium methylate / methanol / 0.5 h / 30 °C View Scheme | |
| Multi-step reaction with 4 steps 1: pyridine / acetonitrile / 0.5 h / -5 - 5 °C 2: pyridine / dichloromethane / 0.5 h / -10 - 0 °C / Inert atmosphere 3: N-ethyl-N,N-diisopropylamine; triethylamine tris(hydrogen fluoride) / toluene / 48 h / 35 - 40 °C 4: sodium methylate / methanol / 0.5 h / 30 °C View Scheme |
-
-
118-00-3
G

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: hydrogenchloride; potassium dihydrogenphosphate; potassium hydroxide / water; dimethyl sulfoxide / 4 h / 58 - 61 °C / pH 7.1-7.2 / Enzymatic reaction 2.1: pyridine / acetonitrile / 0.5 h / -5 - 5 °C 2.2: Reflux 3.1: pyridine / dichloromethane / 0.5 h / -10 - 0 °C / Inert atmosphere 4.1: N-ethyl-N,N-diisopropylamine; triethylamine tris(hydrogen fluoride) / toluene / 48 h / 35 - 40 °C 5.1: sodium methylate / methanol / 0.5 h / 30 °C View Scheme | |
| Multi-step reaction with 5 steps 1: hydrogenchloride; potassium dihydrogenphosphate; potassium hydroxide / water; dimethyl sulfoxide / 4 h / 58 - 61 °C / pH 7.1-7.2 / Enzymatic reaction 2: pyridine / acetonitrile / 0.5 h / -5 - 5 °C 3: pyridine / dichloromethane / 0.5 h / -10 - 0 °C / Inert atmosphere 4: N-ethyl-N,N-diisopropylamine; triethylamine tris(hydrogen fluoride) / toluene / 48 h / 35 - 40 °C 5: sodium methylate / methanol / 0.5 h / 30 °C View Scheme |
-
-
58-96-8
uridine

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: hydrogenchloride; potassium dihydrogenphosphate; potassium hydroxide / water; dimethyl sulfoxide / 4 h / 58 - 61 °C / pH 7.1-7.2 / Enzymatic reaction 2.1: pyridine / acetonitrile / 0.5 h / -5 - 5 °C 2.2: Reflux 3.1: pyridine / dichloromethane / 0.5 h / -10 - 0 °C / Inert atmosphere 4.1: N-ethyl-N,N-diisopropylamine; triethylamine tris(hydrogen fluoride) / toluene / 48 h / 35 - 40 °C 5.1: sodium methylate / methanol / 0.5 h / 30 °C View Scheme | |
| Multi-step reaction with 5 steps 1: hydrogenchloride; potassium dihydrogenphosphate; potassium hydroxide / water; dimethyl sulfoxide / 4 h / 58 - 61 °C / pH 7.1-7.2 / Enzymatic reaction 2: pyridine / acetonitrile / 0.5 h / -5 - 5 °C 3: pyridine / dichloromethane / 0.5 h / -10 - 0 °C / Inert atmosphere 4: N-ethyl-N,N-diisopropylamine; triethylamine tris(hydrogen fluoride) / toluene / 48 h / 35 - 40 °C 5: sodium methylate / methanol / 0.5 h / 30 °C View Scheme |
-
-
14215-97-5
1-O-acetyl-2,3,5-tri-O-benzoyl-D-ribofuranose

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydrogen bromide; tetrabutyl ammonium fluoride / tetrahydrofuran / 3 h / Cooling with ice 1.2: 2 h 2.1: potassium tert-butylate; calcium hydride / acetonitrile; tert-butyl alcohol / 1 h / 60 °C 2.2: 10 h / 60 °C 3.1: sodium methylate; methanol / 2 h / 0 °C View Scheme |
-
-
123318-82-1
clofarabine

-
-
18162-48-6
tert-butyldimethylsilyl chloride

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; | 93% |
-
-
123318-82-1
clofarabine

-
-
58479-61-1
tert-butylchlorodiphenylsilane

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 0 - 20℃; Inert atmosphere; | 91% |
| With 1H-imidazole In N,N-dimethyl-formamide at 0 - 20℃; for 8h; | 4.1 g |
| With 1H-imidazole In N,N-dimethyl-formamide at 0 - 20℃; for 8h; | 4.1 g |
-
-
69304-37-6
1,3-Dichloro-1,1,3,3-tetraisopropyldisiloxane

-
-
123318-82-1
clofarabine

-
A
-
1450815-72-1
2-chloro-9-[2-deoxy-2-fluoro-3,5-O-(1,1,3,3-tetraisopropyldisiloxane-1,3-diyl)-β-D-arabinofuranosyl]adenine

-
B
-
1450815-73-2
2-chloro-9-[2-deoxy-2-fluoro-5-O-(1-hydroxy-1,1,3,3-tetraisopropyldisiloxane-3-yl)-β-D-arabinofuranosyl]adenine

-
C
-
1450815-74-3
C32H48Cl2F2N10O7Si2

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 24h; | A 87% B 9% C 2% |

-
-
123318-82-1
clofarabine

-
-
20187-82-0, 20227-41-2, 64183-27-3, 123334-75-8
9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)adenine

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen; palladium on activated charcoal In ethanol; water at 20℃; for 18h; | 82% |
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; for 48h; | 57% |

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Stage #1: phosphoric acid-(2R-decyloxy-3-dodecyoxo)propyl ester With pyridine; 2,4,6-triisopropylphenylsulfonyl chloride at 20 - 25℃; for 1h; Inert atmosphere; Stage #2: clofarabine for 20h; Inert atmosphere; Stage #3: With sodium methylate In methanol pH=7; Inert atmosphere; | 76% |
-
-
98-88-4
benzoyl chloride

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Stage #1: clofarabine With pyridine; chloro-trimethyl-silane at 0℃; for 0.5h; Stage #2: benzoyl chloride at 20℃; for 24h; | 76% |
-
-
123318-82-1
clofarabine

-
-
98-59-9
p-toluenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With pyridine at 0 - 20℃; for 24h; | 76% |

| Conditions | Yield |
|---|---|
| With pyridine; 1-methyl-1H-imidazole In tetrahydrofuran at -80 - 20℃; for 12.2667h; | 66% |
-
-
1499-29-2
P,P'-methanediyl-bis-phosphonic acid tetrachloride

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Stage #1: P,P'-methanediyl-bis-phosphonic acid tetrachloride; clofarabine With triethyl phosphate at 0℃; Inert atmosphere; Stage #2: | 63% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water at 20℃; for 18h; | 58% |
| With sodium hydroxide In tetrahydrofuran; water at 20℃; for 18h; | 58% |
-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| With lithium hydroxide; dihydrogen peroxide In water at 60℃; for 24h; | 48% |

| Conditions | Yield |
|---|---|
| In methanol at 60℃; for 168h; Inert atmosphere; | 48% |
-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; lithium hydroxide In water at 60℃; for 24h; Inert atmosphere; | 48% |

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Stage #1: phosphoric acid-(2S-decyloxy-3-dodecyloxoy)propyl ester With pyridine; 2,4,6-triisopropylphenylsulfonyl chloride at 20 - 25℃; Inert atmosphere; Stage #2: clofarabine Inert atmosphere; Stage #3: With sodium methylate In methanol pH=7; Inert atmosphere; | 28% |
-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dimethyl sulfoxide at 54℃; for 3.5h; Inert atmosphere; | 25% |

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate); triethylamine In tetrahydrofuran at 20℃; for 12h; | 15% |
-
-
134217-15-5
2-fluoro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)-9H-purin-6-amine

-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 80℃; for 1.9h; Inert atmosphere; | 0.8% |
-
-
123318-82-1
clofarabine

-
A
-
1839-18-5
2-chloroadenine

-
B
-
850883-62-4
2-deoxy-2-fluoro-α-D-arabinofuranose 1-phosphate

| Conditions | Yield |
|---|---|
| With E. coli purine nucleoside phosphorylase Enzyme kinetics; Substitution; |
-
-
123318-82-1
clofarabine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 82 percent / H2; NH3 / Pd/C / ethanol; H2O / 18 h / 20 °C 2.1: pyridine / 18 h / 20 °C 2.2: 72 percent / pyridine / 18 h / 20 °C View Scheme |
Clofarabine Specification
The Clofarabine, with the CAS registry number 123318-82-1,is also known as 5-(6-Amino-2-chloro-purin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol . It belongs to the product categories of Nucleotides;Pharmaceutical intermediate. This chemical's molecular formula is C10H11ClFN5O3 and molecular weight is 303.68. What's more,Its systematic name is 2-Chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)-9H-purin-6-amine .It is a White Solid which is approved in the United States for treatment of acute lymphoblastic leukaemia in paediatric patients who have relapsed.
Physical properties about Clofarabine are:
(1)ACD/LogP: 0.446; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.45; (4)ACD/LogD (pH 7.4): 0.45; (5)ACD/BCF (pH 5.5): 1.29; (6)ACD/BCF (pH 7.4): 1.29; (7)ACD/KOC (pH 5.5): 41.66; (8)ACD/KOC (pH 7.4): 41.67; (9)#H bond acceptors: 8; (10)#H bond donors: 4; (11)#Freely Rotating Bonds: 4; (12)Index of Refraction: 1.844; (13)Molar Refractivity: 63.578 cm3; (14)Molar Volume: 143.096 cm3; (15)Polarizability: 25.204 10-24cm3 ; (16)Surface Tension: 86.3119964599609 dyne/cm; (17)Density: 2.122 g/cm3; (18)Flash Point: 316.379 °C; (19)Enthalpy of Vaporization: 93.858 kJ/mol; (20)Boiling Point: 599.522 °C at 760 mmHg; (21)Vapour Pressure: 0 mmHg at 25°C.
Structure Descriptors of Clofarabine:
(1)Clc1nc(c2ncn(c2n1)[C@@H]3O[C@@H]([C@@H](O)[C@@H]3F)CO)N;
(2)Std. InChI:InChI=1S/C10H11ClFN5O3/c11-10-15-7(13)5-8(16-10)17(2-14-5)9-4(12)6(19)3(1-18)20-9/h2-4,6,9,18-19H,1H2,(H2,13,15,16)/t3-,4+,6-,9-/m1/s1;
(3)Std. InChIKey:WDDPHFBMKLOVOX-AYQXTPAHSA-N.
Uses of Clofarabine:
Clofarabine is second generation purine nucleoside analog; antimetabolite that inhibits DNA synthesis and resists deamination by adenosine deaminase, and is also antineoplastic. It is FDA-approved for treating relapsed or refractory acute lymphoblastic leukaemia (ALL) in children after at least two other types of treatment have failed. Furthermore, Clofarabine displays greater affinity towards dCK (deoxycytidine kinase, dCyd), an enzyme essential in nucleoside phosphorylation. As a intermediate, it can be used to manufacture antineoplastic Clofarabine.
Side effects of Clofarabine
(1)Side effects on tumor lysis syndrome (TLS): TLS is very serious and can lead to death if it is not treated right away.
Clofarabine can quickly kills leukaemia cells in the blood. Then the body may react to this. Symptoms include fast breathing, fast heartbeat, low blood pressure, and fluid in the lungs.
(2)Side effects on Bone marrow problems (suppression). Clofarabine can stop the bone marrow from making enough red blood cells, white blood cells, and platelets. Serious side effects that can happen because of bone marrow suppression include severe infection (sepsis), bleeding, and anemia.
(3)Effects on pregnancy and breastfeeding. Girls and women should not become pregnant or breastfeed during treatment which harm the baby. (4)Effects on Dehydration and low blood pressure. Clofarabine (CAS NO.123318-82-1) can cause vomiting and diarrhea which may lead to low body fluid (dehydration). Signs and symptoms of dehydration include dizziness, lightheadedness, fainting spells, or decreased urination.
(5)Other side effects. The most common side effects are stomach problems (including vomiting, diarrhea, and nausea), and effects on blood cells (including low red blood cells count, low white blood cell count, low platelet count, fever, and infection. Clofarabine can also cause tachycardia and can affect the liver and kidneys.
Production of Clofarabine
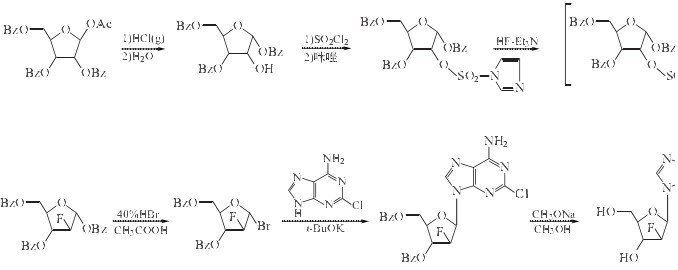
The reaction flask was added 2-chloro-9-(2-deoxy-2-fluoro-3,5-di-O-benzoyl-beta-D arabinose yl) adenine 1.5g (3mmol) and methanol 40ml,mixed with stirring. Then it was added sodium methoxide, 0.05g (content> 50%), the reaction was stirred for 40min. Then the mixture was cooled to room temperature, adjusted to pH 7 with acetic acid, filtered, and the filter cake was washed with an ice-methanol 10ml, added to the methanol 40ml, and heated to 63 °C, and then cooled to -10 o C. Still 1h, filtered, and the filter cake was washed with an ice-methanol 10ml, drained, dried under reduced pressure to give an off-white powdery solid clofarabine 0.48g. The yield is 54%.
Related Products
- Clofarabine
- 123-31-9
- 123319-30-2
- 123-32-0
- 123324-71-0
- 123-33-1
- 1233323-55-1
- 123333-53-9
- 123333-55-1
- 123333-60-8
- 123333-66-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View