-
Name
Clomethiazole
- EINECS 208-565-7
- CAS No. 533-45-9
- Article Data19
- CAS DataBase
- Density 1.219 g/cm3
- Solubility
- Melting Point 127-128 °C
- Formula C6H8ClNS
- Boiling Point 245.774 °C at 760 mmHg
- Molecular Weight 161.655
- Flash Point 102.441 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 4-Methyl-5-(b-chloroethyl)thiazole;5-(2-Chloroethyl)-4-methylthiazole;5-(b-Chloroethyl)-4-methylthiazole;Chlorethiazol;Chlorethiazole;Chlormethiazol;Chlormethiazole;Clomethiazole;Clomethiazolum;Distraneurin;Emineurina;Somnevrin;
- PSA 41.13000
- LogP 3.03480
Synthetic route

| Conditions | Yield |
|---|---|
| With thionyl chloride for 3h; Reflux; | 98% |
| With thionyl chloride for 2h; Heating; | 96% |
| With phosphorus pentachloride In dichloromethane for 3h; Heating; | 95% |

-
-
533-45-9
chlormethiazole

| Conditions | Yield |
|---|---|
| With hydrogenchloride; dihydrogen peroxide |
-
-
853261-35-5
5-(2-ethoxyethyl)-4-methylthiazole

-
-
533-45-9
chlormethiazole

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
663179-27-9
5-ethoxy-3-chloro-pentan-2-one

-
-
533-45-9
chlormethiazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: concentrated aqueous HCl View Scheme |
-
-
663179-24-6
2-(2-ethoxy-ethyl)-2-chloro-acetoacetic acid ethyl ester

-
-
533-45-9
chlormethiazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: glacial acetic acid; H2SO4 3: concentrated aqueous HCl View Scheme |
-
-
13045-13-1
3-chloro-3-acetylpropanol

-
-
533-45-9
chlormethiazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ethanol 2: concentrated aqueous HCl View Scheme |
-
-
58371-98-5
3, 5-dichloro-2-pentanone

-
-
533-45-9
chlormethiazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ethanol / anschliessend Behandeln mit H2O 2: H2O2; concentrated aqueous HCl View Scheme |
-
-
773837-37-9
sodium cyanide

-
-
533-45-9
chlormethiazole

-
-
6469-34-7
3-(4-methyl-thiazol-5-yl)propionitrile

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20 - 80℃; for 8h; | 98% |
-
-
533-45-9
chlormethiazole

-
-
77-78-1
dimethyl sulfate

-
-
78919-46-7
5-(β-Chloroethyl)-3,4-dimethylthiazolium methyl sulfate

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 24h; | 85% |
-
-
533-45-9
chlormethiazole

-
-
1074-82-4
potassium phtalimide

-
-
36956-91-9
4-methyl-5-(β-phthalimidoethyl)thiazole

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100 - 105℃; for 2h; | 67% |
| In N,N-dimethyl-formamide (heating); |
-
-
533-45-9
chlormethiazole

-
-
330436-68-5
5-(2-chloroethyl)-2-iodo-4-methylthiazole

| Conditions | Yield |
|---|---|
| Stage #1: chlormethiazole With methyllithium In diethyl ether at -40℃; Stage #2: With iodine In diethyl ether at -40 - 20℃; Further stages.; | 63% |
-
-
533-45-9
chlormethiazole

-
-
151-50-8
potassium cyanide

-
-
6469-34-7
3-(4-methyl-thiazol-5-yl)propionitrile

| Conditions | Yield |
|---|---|
| In ethanol; water at 70℃; for 24h; | 60% |
-
-
533-45-9
chlormethiazole

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile Reflux; | 39% |
-
-
533-45-9
chlormethiazole

-
-
1002110-68-0
N-(3-Chlorobenzyl)-1H-indazole-6-carboxamide

-
-
1002109-98-9
N-(3-Chlorobenzyl)-2-[2-(4-methyl-1,3-thiazol-5-yl)ethyl]-2H-indazole-6-carboxamide

| Conditions | Yield |
|---|---|
| With caesium carbonate; sodium iodide In N,N-dimethyl-formamide at 50℃; for 16h; Inert atmosphere; | 8% |
-
-
533-45-9
chlormethiazole

-
-
111-42-2
2,2'-iminobis[ethanol]

-
-
99977-12-5
bis-(2-hydroxy-ethyl)-[2-(4-methyl-thiazol-5-yl)-ethyl]-amine

| Conditions | Yield |
|---|---|
| With ethanol |

| Conditions | Yield |
|---|---|
| With benzene |
-
-
110-89-4
piperidine

-
-
533-45-9
chlormethiazole

-
-
36956-90-8
1-[2-(4-methyl-thiazol-5-yl)-ethyl]-piperidine

| Conditions | Yield |
|---|---|
| In butanone Heating; |
-
-
110-91-8
morpholine

-
-
533-45-9
chlormethiazole

-
-
36958-88-0
4-[2-(4-methyl-thiazol-5-yl)-ethyl]-morpholine

| Conditions | Yield |
|---|---|
| In butanone (heating); |
-
-
533-45-9
chlormethiazole

-
-
74-93-1
methylthiol

-
-
33121-10-7
4-methyl-5-(2-methylsulfanyl-ethyl)-thiazole

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl-formamide Ambient temperature; |
-
-
533-45-9
chlormethiazole

-
-
109-89-7
diethylamine

-
-
95164-46-8
diethyl-[2-(4-methyl-thiazol-5-yl)-ethyl]-amine

| Conditions | Yield |
|---|---|
| In nitrobenzene (heating); |

| Conditions | Yield |
|---|---|
| With sodium ethanolate (heating); |

| Conditions | Yield |
|---|---|
| In acetone Ambient temperature; |
-
-
533-45-9
chlormethiazole

-
-
107814-79-9
clomethiazol-I2

| Conditions | Yield |
|---|---|
| With iodine In tetrachloromethane at 20℃; Thermodynamic data; ΔH0, ΔS0, ΔG0; |
-
-
533-45-9
chlormethiazole

-
-
330436-76-5
5-(2-chloroethyl)-2-hex-1-ynyl-4-methylthiazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: MeLi / diethyl ether / -40 °C 1.2: 63 percent / I2 / diethyl ether / -40 - 20 °C 2.1: 84 percent / Et3N; Pd(PPh3)2Cl2; CuI / CH2Cl2 / 6 h / 65 °C View Scheme |
-
-
533-45-9
chlormethiazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: MeLi / diethyl ether / -40 °C 1.2: 63 percent / I2 / diethyl ether / -40 - 20 °C 2.1: 84 percent / Et3N; Pd(PPh3)2Cl2; CuI / CH2Cl2 / 6 h / 65 °C 3.1: 79 percent / (η6-C6H5BPh3)-Rh+(1,5-COD); (PhO)3P; H2 / CH2Cl2 / 18 h / 110 °C / 15961.1 Torr View Scheme |
-
-
533-45-9
chlormethiazole

-
-
71114-46-0
N-Methyl-N-<(Z)-1-(2-thietanylidene)ethyl>formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / acetone / 24 h / 20 °C 2: 78 percent / 1 M NaOH / trichloroethene / 0.17 h / Ambient temperature; other 5-ω-chloroalkyl-3-methylthiazolium methosulfates View Scheme |
-
-
533-45-9
chlormethiazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: H2O2; aqueous NaOH View Scheme |
-
-
533-45-9
chlormethiazole

-
-
99548-90-0
bis-(2-chloro-ethyl)-[2-(4-methyl-thiazol-5-yl)-ethyl]-amine; dihydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ethanol 2: HCl; CHCl3 / anschliessend Behandeln mit SOCl2 in CHCl3 View Scheme |
Clomethiazole Chemical Properties
5-(2-chloroethyl)-4-methylthiazole (533-45-9) is also named as clomethiazole;chlormethiazole;;ChlormethiazoleHCl;5-(2-Chlorethyl)-4-methylthiazole hydrochloride;Chlorethiazole;Clomethiazolum,and so on.
CAS: 533-45-9
Molecular Formula: C6H8ClNS
Molecular Weight: 161.65
Molecular structure: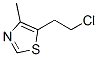
EINECS: 208-565-7
Index of Refraction: 1.545
Molar Refractivity: 41.95 cm3
Molar Volume: 132.6 cm3
Polarizability: 16.63 10-24cm3
Surface Tension: 41.8 dyne/cm
Density: 1.218 g/cm3
Flash Point: 102.4 °C
Enthalpy of Vaporization: 46.33 kJ/mol
Boiling Point: 245.8 °C at 760 mmHg
Vapour Pressure: 0.0442 mmHg at 25°C
Clomethiazole Uses
Clomethiazole Toxicity Data With Reference
| 1. | orl-mus LD50:2110 mg/kg | JMCMAR Journal of Medicinal Chemistry. 7 (1964),167. | ||
| 2. | ipr-mus LD50:190 mg/kg | APSXAS Acta Pharmaceutica Suecdca. 8 (1971),39. | ||
| 3. | ivn-mus LD50:94 mg/kg | APSXAS Acta Pharmaceutica Suecdca. 7 (1970),423. |
RTECS: XJ3850000
Clomethiazole Safety Profile
Related Products
- Clomethiazole
- Clomethiazole ethane-1,2-disulfonate
- 5334-59-8
- 53347-39-0
- 53347-40-3
- 53348-04-2
- 53348-05-3
- 53348-92-8
- 53-34-9
- 533-49-3
- 53-35-0
- 53350-26-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View