-
Name
Clozapine
- EINECS 227-313-7
- CAS No. 5786-21-0
- Article Data19
- CAS DataBase
- Density 1.319 g/cm3
- Solubility ethanol: 1 mg/mL
- Melting Point 182-185 °C
- Formula C18H19ClN4
- Boiling Point 489.159 °C at 760 mmHg
- Molecular Weight 326.829
- Flash Point 249.635 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance Yellow Crystalline Solid
- Safety 26
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Clorazil;Fazaclo;Iprox;Clozapina [INN-Spanish];8-Chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo(b,e)(1,4)diazepine;5H-Dibenzo(b,e)(1,4)diazepine, 8-chloro-11-(4-methyl-1-piperazinyl)-;Leponex;Asaleptin;Clozaril (TN);Clozapine [USAN:BAN:INN];8-chloro-11(4-methy-1-piperaziong)-5-H-dibenzo[b,e][1.4]diazepine;
- PSA 30.87000
- LogP 3.17210
Synthetic route

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| With hydrogenchloride; titanium(III) chloride In water; acetonitrile at 20℃; for 1h; | 97% |
-
-
109-01-3
1-methyl-piperazine

-
-
50892-62-1
8-chloro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In 1,4-dioxane; toluene for 2h; Heating; | 88% |
| With titanium tetrachloride | 53% |
| With hydrogenchloride; titanium tetrachloride In water; methoxybenzene; isopropyl alcohol; toluene | |
| With titanium tetrachloride |

-
-
4407-36-7
(2E)-3-phenyl-2-propen-1-ol

-
A
-
63157-81-3
1-phenyl-propane-1,2,3-triol

-
B
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| With osmium(VIII) oxide In water; tert-butyl alcohol at 20℃; for 19h; Inert atmosphere; | A 73% B 84% |

-
-
65514-72-9
N-(4-chloro-2-nitrophenyl)-N-{2-[(4-methylpiperazin-1-yl)carbonyl]phenyl}amine

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| With tributylphosphine at 150℃; for 48h; Temperature; Reagent/catalyst; | 80% |
| Multi-step reaction with 2 steps 1: 3 h / 150 °C 2: triphenylphosphine / 230 °C View Scheme |

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| With triphenylphosphine at 230℃; | 65% |

| Conditions | Yield |
|---|---|
| With osmium(VIII) oxide In water; tert-butyl alcohol at 20℃; for 19h; Inert atmosphere; | A 8% B 53% |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: o-xylene / Dean-Stark 1.2: Reflux 2.1: iron; calcium chloride / water / 90 - 100 °C 3.1: triethylamine / dichloromethane / 0.33 h / 20 °C 3.2: 2.33 h / 20 °C 4.1: titanium tetrachloride / o-xylene / 10 - 15 °C / Reflux 5.1: titanium tetrachloride / 10 °C / Reflux View Scheme | |
| Multi-step reaction with 6 steps 1.2: 12 h / 20 °C / Reflux 2.1: iodine / 3 h / 50 - 55 °C 3.1: sodium dithionate / methanol / 2 h / 50 - 55 °C 4.1: toluene / 0 - 5 °C 5.1: trichlorophosphate / 3 h / Reflux 6.1: potassium carbonate / ethanol / 3 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.2: 12 h / 20 °C / Reflux 2.1: iodine / 3 h / 50 - 55 °C 3.1: sodium dithionate / methanol / 2 h / 50 - 55 °C 4.1: toluene / 0 - 5 °C 5.1: trichlorophosphate / 3 h / Reflux 6.1: potassium carbonate / ethanol / 3 h / Reflux View Scheme |
-
-
16611-15-7
N-(4-chloro-2-nitrophenyl)benzenamine

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: iodine / 3 h / 50 - 55 °C 2: sodium dithionate / methanol / 2 h / 50 - 55 °C 3: toluene / 0 - 5 °C 4: trichlorophosphate / 3 h / Reflux 5: potassium carbonate / ethanol / 3 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: o-xylene / Dean-Stark 1.2: Reflux 2.1: iron; calcium chloride / water / 90 - 100 °C 3.1: triethylamine / dichloromethane / 0.33 h / 20 °C 3.2: 2.33 h / 20 °C 4.1: titanium tetrachloride / o-xylene / 10 - 15 °C / Reflux 5.1: titanium tetrachloride / 10 °C / Reflux View Scheme |

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium dithionate / methanol / 2 h / 50 - 55 °C 2: toluene / 0 - 5 °C 3: trichlorophosphate / 3 h / Reflux 4: potassium carbonate / ethanol / 3 h / Reflux View Scheme |

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: toluene / 0 - 5 °C 2: trichlorophosphate / 3 h / Reflux 3: potassium carbonate / ethanol / 3 h / Reflux View Scheme |

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trichlorophosphate / 3 h / Reflux 2: potassium carbonate / ethanol / 3 h / Reflux View Scheme |

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol for 3h; Reflux; | 5 g |

| Conditions | Yield |
|---|---|
| With C17H24N5Ru(1+)*F6P(1-); potassium acetate; potassium carbonate In 1-methyl-pyrrolidin-2-one at 35℃; for 48h; Inert atmosphere; | 96% |
-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| Stage #1: clozaril With 3-chloro-benzenecarboperoxoic acid In methanol at 23℃; for 0.166667h; Inert atmosphere; Stage #2: With triethylamine In methanol for 0.166667h; Inert atmosphere; | 94% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 0.166667h; |
-
-
36016-40-7
mesitylenesulfonylhydroxylamine

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.333333h; | 82% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide at 20℃; | 80% |

| Conditions | Yield |
|---|---|
| With nickel(II) acetylacetonate; potassium fluoride; phenylsilane; ammonia; zinc; bis(2-diphenylphosphinoethyl)phenylphosphine In 1-methyl-pyrrolidin-2-one at 90 - 140℃; under 760.051 Torr; for 20h; Sealed tube; | 80% |
-
-
82626-48-0
N,N,6-trimethyl-2-(4-methylphenyl)imidazo[1,2-a]pyridine-3-acetamide

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| With C17H24N5Ru(1+)*F6P(1-); potassium acetate; potassium carbonate In 1-methyl-pyrrolidin-2-one at 35℃; for 144h; Reagent/catalyst; Inert atmosphere; | 76% |
| With C17H24N5Ru(1+)*F6P(1-); potassium acetate; potassium carbonate In 1-methyl-pyrrolidin-2-one at 35℃; for 144h; Inert atmosphere; Glovebox; | 76% |

| Conditions | Yield |
|---|---|
| Stage #1: clozaril With carbonochloridic acid 1-chloro-ethyl ester In 1,2-dicholoroethane at 0℃; for 22.5h; Inert atmosphere; Reflux; Stage #2: With methanol at 50℃; for 2h; Inert atmosphere; | 69% |
| Stage #1: clozaril With carbonochloridic acid 1-chloro-ethyl ester In 1,2-dichloro-ethane for 24h; Reflux; Stage #2: With methanol at 50℃; for 2h; | |
| With cytochrome P450 1A2; cytochrome P450 3A4 |
-
-
5786-21-0
clozaril

-
-
73183-34-3
bis(pinacol)diborane

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); potassium acetate; XPhos In 1,4-dioxane at 110℃; for 12h; Inert atmosphere; Sealed tube; | 59% |

| Conditions | Yield |
|---|---|
| In acetone at 20℃; | 55% |
-
-
112-13-0
n-decanoyl chloride

-
-
5786-21-0
clozaril

-
-
1357146-74-7
1-[8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepin-5-yl]decan-1-one

| Conditions | Yield |
|---|---|
| With pyridine; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 2h; Inert atmosphere; | 36% |

| Conditions | Yield |
|---|---|
| Stage #1: clozaril With ethylenediaminetetraacetic acid trisodium salt; oxygen; manganese (II) acetate tetrahydrate; ascorbic acid In water pH=4; Udenfriend reaction; Stage #2: With ferrous(II) sulfate heptahydrate; ethylenediaminetetraacetic acid trisodium salt; oxygen In water at 45℃; for 2h; Udenfriend reaction; | A 4.93% B 2.98% |

| Conditions | Yield |
|---|---|
| With horseradish peroxidase type VI; dihydrogen peroxide In phosphoric acid at 25℃; for 4h; pH=7.4; Oxidation; Enzymatic reaction; | |
| With sodium hypochlorite In ethanol; water Oxidation; |

-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| With hypochloric acid In water at 25℃; pH=6; Kinetics; Oxidation; |

| Conditions | Yield |
|---|---|
| Stage #1: N-acetylcystein; clozaril With sodium hypochlorite In ethanol for 0.166667h; pH=4.0; Stage #2: 1-hydroxy-pyrrolidine-2,5-dione With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In N,N-dimethyl-formamide; acetonitrile Stage #3: keyhole limpet hemocyanin In phosphate buffer; acetonitrile at 20℃; for 1h; pH=8.0; |


| Conditions | Yield |
|---|---|
| Stage #1: N-acetylcystein; clozaril With sodium hypochlorite In ethanol for 0.166667h; pH=4.0; Stage #2: 1-hydroxy-pyrrolidine-2,5-dione With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In N,N-dimethyl-formamide; acetonitrile Stage #3: rabbit serum albumin In phosphate buffer; acetonitrile at 20℃; for 1h; pH=8.0; |

-
-
5786-21-0
clozaril

-
-
156632-03-0
8-chloro-11-(4-methylpiperazin-1-yl)-5-nitroso-5H-dibenzo[b,e][1,4]diazepine

| Conditions | Yield |
|---|---|
| With isopentyl nitrite In dichloromethane | |
| With isopentyl nitrite In dichloromethane at 20℃; for 3h; |
-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| With isopentyl nitrite In dichloromethane at 20℃; for 3h; |
-
-
5786-21-0
clozaril

-
-
136772-01-5
8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepin-5-amine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: isoamyl nitrite / CH2Cl2 2: AcOH; Zn View Scheme | |
| Multi-step reaction with 2 steps 1: isoamyl nitrite / CH2Cl2 / 3 h / 20 °C 2: 0.84 g / Zn; AcOH / 3 h View Scheme | |
| Multi-step reaction with 2 steps 1: isopentyl nitrite / dichloromethane / 3 h / 20 °C 2: acetic acid; zinc / 4 h / 10 - 15 °C View Scheme | |
| Stage #1: clozaril With isopentyl nitrite In dichloromethane at 27℃; for 3h; Stage #2: With acetic acid; zinc at 10 - 15℃; for 1h; |
-
-
5786-21-0
clozaril

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: isoamyl nitrite / CH2Cl2 / 3 h / 20 °C 2.1: 0.84 g / Zn; AcOH / 3 h 3.1: Argopore-CHO resin; NaBH3CN; AcOH 3.2: Et3N 3.3: TFA View Scheme |
Clozapine Chemical Properties
Empirical Formula: C18H19ClN4
Molecular Weight: 326.8233 g/mol
EINECS: 227-313-7
Index of Refraction: 1.681
Density: 1.31 g/cm3
Flash Point: 249.6 °C
Melting point: 182-185 °C
Solubility: Ethanol: 1 mg/mL
Appearance: Yellow crystalline solid
Enthalpy of Vaporization: 75.54 kJ/mol
Boiling Point: 489.2 °C at 760 mmHg
Vapour Pressure: 1.02E-09 mmHg at 25 °C
Structure of Clozapine (CAS NO.5786-21-0):
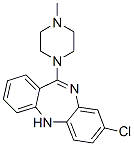
IUPAC Name: 3-Chloro-6-(4-methylpiperazin-1-yl)-5H-benzo[c][1,5]benzodiazepine
Product Category: Active Pharmaceutical Ingredients;Intermediates & Fine Chemicals;Dopamine receptor
Clozapine History
Clozapine (CAS NO.5786-21-0) by Sandoz in 1961, and introduced in Europe in 10 years. In 1975, the report of agranulocytosis leading to death in some patients treated with clozapine, clozapine manufacturers to voluntarily withdraw. Clozapine fell out of favor for more than a decade. However, studies have shown that clozapine is more effective for refractory schizophrenia than other antipsychotics, the U.S. Food and Drug Administration and health authorities in most other countries approved its use only for refractory schizophrenia Patients require regular blood tests to monitor the neutrophil, and then agranuloctytosis development. December 2002, clozapine was also approved to reduce the risk of suicide in patients with schizophrenia or schizoaffective is considered in the risk of suicide.
Clozapine Uses
Clozapine (CAS NO.5786-21-0) can be used as an antipsychotic.
Clozapine Production
Clozapine (CAS NO.5786-21-0) can be obtained from the reaction of 2 - Amino -4 - chloro-aniline 2 -2 '- carboxylic acid (4''-methyl) piperazine with Phosphorus oxychloride .
Clozapine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | oral | 4762ug/kg (4.762mg/kg) | BEHAVIORAL: ATAXIA BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) BEHAVIORAL: ANTIPSYCHOTIC | American Journal of Emergency Medicine. Vol. 14, Pg. 462, 1996. |
| dog | LD50 | oral | 145mg/kg (145mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING | Farmaco, Edizione Pratica. Vol. 26, Pg. 585, 1971. |
| guinea pig | LD50 | oral | 510mg/kg (510mg/kg) | Farmaco, Edizione Pratica. Vol. 26, Pg. 585, 1971. | |
| human | TDLo | oral | 5mg/kg/7D-I (5mg/kg) | CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 919, 1972. |
| man | TDLo | oral | 4286ug/kg/11D (4.286mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" BLOOD: CHANGES IN CELL COUNT (UNSPECIFIED) | Journal of Clinical Pyschopharmacology. Vol. 13, Pg. 155, 1993. |
| man | TDLo | oral | 5357ug/kg/5D- (5.357mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" BEHAVIORAL: ANTIPSYCHOTIC CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP | Journal of Clinical Pyschopharmacology. Vol. 12, Pg. 139, 1992. |
| man | TDLo | oral | 27mg/kg/13D-I (27mg/kg) | AUTONOMIC NERVOUS SYSTEM: "SMOOTH MUSCLE RELAXANT (MECHANISM UNDEFINED, SPASMOLYTIC)" BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" BEHAVIORAL: ATAXIA | Journal of Clinical Psychiatry. Vol. 55, Pg. 38, 1994. |
| man | TDLo | oral | 40mg/kg (40mg/kg) | BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION | Veterinary and Human Toxicology. Vol. 41, Pg. 20, 1999. |
| man | TDLo | oral | 86mg/kg/15D-I (86mg/kg) | CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | British Journal of Psychiatry. Vol. 173, Pg. 440, 1998. |
| man | TDLo | oral | 270mg/kg/6W-I (270mg/kg) | LUNGS, THORAX, OR RESPIRATION: STRUCTURAL OR FUNCTIONAL CHANGE IN TRACHEA OR BRONCHI BLOOD: AGRANULOCYTOSIS GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Annales de Medecine Interne. Vol. 144, Pg. 494, 1993. |
| man | TDLo | oral | 429mg/kg/60D- (429mg/kg) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) | American Journal of Psychiatry. Vol. 152, Pg. 649, 1995. |
| man | TDLo | oral | 429mg/kg (429mg/kg) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP BEHAVIORAL: COMA | Journal of Toxicology, Clinical Toxicology. Vol. 38, Pg. 325, 2000. |
| man | TDLo | oral | 2211mg/kg/37W (2211mg/kg) | BLOOD: "CHANGES IN SERUM COMPOSITION (E.G., TP, BILIRUBIN, CHOLESTEROL)" | Journal of Clinical Pyschopharmacology. Vol. 15, Pg. 287, 1995. |
| man | TDLo | oral | 3280mg/kg/82W (3280mg/kg) | BLOOD: AGRANULOCYTOSIS | American Journal of Psychiatry. Vol. 153, Pg. 1503, 1996. |
| mouse | LD50 | intraperitoneal | 90mg/kg (90mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Farmaco, Edizione Pratica. Vol. 26, Pg. 585, 1971. |
| mouse | LD50 | intravenous | 36500ug/kg (36.5mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Kiso to Rinsho. Clinical Report. Vol. 7, Pg. 667, 1973. |
| mouse | LD50 | oral | 150mg/kg (150mg/kg) | Journal of Medicinal Chemistry. Vol. 23, Pg. 878, 1980. | |
| mouse | LD50 | subcutaneous | 194mg/kg (194mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: EXCITEMENT BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Kiso to Rinsho. Clinical Report. Vol. 7, Pg. 667, 1973. |
| rat | LD50 | intramuscular | 210mg/kg (210mg/kg) | Farmaco, Edizione Pratica. Vol. 26, Pg. 585, 1971. | |
| rat | LD50 | intravenous | 41600ug/kg (41.6mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION BEHAVIORAL: ATAXIA | Kiso to Rinsho. Clinical Report. Vol. 7, Pg. 667, 1973. |
| rat | LD50 | oral | 251mg/kg (251mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 7, Pg. 667, 1973. | |
| rat | LD50 | subcutaneous | 240mg/kg (240mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Kiso to Rinsho. Clinical Report. Vol. 7, Pg. 667, 1973. |
| women | TDLo | oral | 20mg/kg (20mg/kg) | BEHAVIORAL: COMA BLOOD: LEUKOPENIA GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF ENDOCRINE PANCREAS | Annals of Internal Medicine. Vol. 121, Pg. 722, 1994. |
| women | TDLo | oral | 28mg/kg/2W-I (28mg/kg) | GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF ENDOCRINE PANCREAS | Lancet. Vol. 340, Pg. 251, 1992. |
| women | TDLo | oral | 96mg/kg/48D-I (96mg/kg) | BLOOD: LEUKOPENIA BLOOD: AGRANULOCYTOSIS | Human Psychopharmacology. Vol. 13, Pg. 583, 1998. |
| women | TDLo | oral | 104mg/kg/26D- (104mg/kg) | BLOOD: EOSINOPHILIA | Journal of Clinical Psychiatry. Vol. 59, Pg. 195, 1998. |
| women | TDLo | oral | 168mg/kg/4W-I (168mg/kg) | BLOOD: AGRANULOCYTOSIS | Netherlands Journal of Medicine. Vol. 52, Pg. 26, 1998. |
| women | TDLo | oral | 280mg/kg/5W-I (280mg/kg) | LIVER: "HEPATITIS (HEPATOCELLULAR NECROSIS), DIFFUSE" BLOOD: EOSINOPHILIA | American Journal of Psychiatry. Vol. 150, Pg. 985, 1993. |
Clozapine Safety Profile
Hazard Codes:  Xn,
Xn, Xi
Xi
Risk Statements: 22-36/37/38
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
Clozapine Specification
Clozapine , its cas register number is 5786-21-0. It also can be called 8-Chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine ; Leponex ; and 5H-Dibenzo(b,e)(1,4)diazepine, 8-chloro-11-(4-methyl-1-piperazinyl)- . Many minor though some serious and potentially fatal: the more common include constipation, drooling, muscle stiffness, sedation, tremors, orthostasis, hyperglycemia, and weight gain. Extrapyramidal symptoms may subside somewhat after a person switches from another antipsychotic to Clozapine. Clozapine (CAS NO.5786-21-0) also carries black box warnings for seizures, myocarditis, and "other adverse cardiovascular and respiratory effects." Slow titration of dosing may also decrease the risk for orthostatic hypotension and other adverse cardiovascular side effects. It may have a synergistic effect with the sedating action of other drugs such as benzodiazepines, and thus respiratory depression may result with concomitant use. Care should be taken, especially if the latter drugs are given parenterally.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View