-
Name
Crotonic anhydride
- EINECS 210-807-1
- CAS No. 623-68-7
- Article Data24
- CAS DataBase
- Density 1.038 g/cm3
- Solubility
- Melting Point 72oC
- Formula C8H10O3
- Boiling Point 247 °C at 760 mmHg
- Molecular Weight 154.166
- Flash Point 106.1 °C
- Transport Information
- Appearance
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 2-Butenoicacid, anhydride (9CI);Crotonic anhydride (6CI,7CI,8CI);2-Butenoic anhydride;Crotonic acid anhydride;NSC 97397;Crotonic Anhydride;
- PSA 43.37000
- LogP 1.20840
Synthetic route

| Conditions | Yield |
|---|---|
| With 2-chloro-1,3-dimethylimidazolinium chloride; triethylamine In dichloromethane at 20℃; for 18h; Acylation; | 96% |
| With dicyclohexyl-carbodiimide |

| Conditions | Yield |
|---|---|
| With 1,4-dioxane at 70 - 80℃; folgendes Destillieren des Reaktionsgemisches; |

| Conditions | Yield |
|---|---|
| With acetic anhydride |

| Conditions | Yield |
|---|---|
| With dichloromethane; mercury(II) oxide |
-
-
10487-71-5
crotonyl chloride

-
-
121-44-8
triethylamine

-
-
71-43-2
benzene

-
-
623-68-7
trans-crotonic anhydride

-
-
60-29-7
diethyl ether

-
-
107-93-7
(E)-but-2-enoic acid

-
-
927-80-0
1-ethoxyacetylene

-
-
623-68-7
trans-crotonic anhydride


| Conditions | Yield |
|---|---|
| With diethyl ether |

| Conditions | Yield |
|---|---|
| With oxygen; cobalt(II) acetate; acetic acid at 30℃; unter leichtem Ueberdruck; | |
| With oxygen; copper (I) acetate; acetic acid at 30℃; unter leichtem Ueberdruck; | |
| With oxygen; manganese(II) acetate; acetic acid at 30℃; unter leichtem Ueberdruck; | |
| With oxygen; acetic acid at 30℃; in Gegenwart einer Mischung von Wismut-,Kobalt- und Kupferpulver; |
-
-
625-35-4, 3488-22-0, 10487-71-5
trans-chrotonyl chloride

-
-
623-68-7
trans-crotonic anhydride

-
-
623-68-7
trans-crotonic anhydride

| Conditions | Yield |
|---|---|
| With thionyl chloride; Petroleum ether |
-
-
623-68-7
trans-crotonic anhydride

-
-
366801-82-3
methyl 2-O-benzyl-4,6-di-O-methyl-α-D-mannopyranoside

-
-
366801-85-6
methyl 2-O-benzyl-3-O-crotonyl-4,6-di-O-methyl-α-D-mannopyranoside

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 16h; | 100% |
-
-
623-68-7
trans-crotonic anhydride

- polymer, product of radical polymerization, loading of chiral auxiliary 2.42 mmol/g, Mn=5800, Mw=7000 (GPC, polystyrene standards); monomer(S): styrene; (4S)-4-{4-[(4-vinylbenzyl)oxy]benzyl}-1,3-oxazolidin-2-one
-
polymer, product of radical polymerization, loading of chiral auxiliary 2.42 mmol/g, Mn=5800, Mw=7000 (GPC, polystyrene standards); monomer(S): styrene; (4S)-4-{4-[(4-vinylbenzyl)oxy]benzyl}-1,3-oxazolidin-2-one

- polymer, loading of chiral auxiliary 2.42 mmol/g, Mn=7500, Mw=10000 (GPC, polystyrene standards); monomer(s): styrene; (4S)-4-{4-[(4-vinylbenzyl)oxy]benzyl}-1,3-oxazolidin-2-one; trans-crotonic anhydride
-
polymer, loading of chiral auxiliary 2.42 mmol/g, Mn=7500, Mw=10000 (GPC, polystyrene standards); monomer(s): styrene; (4S)-4-{4-[(4-vinylbenzyl)oxy]benzyl}-1,3-oxazolidin-2-one; trans-crotonic anhydride

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In tetrahydrofuran at 70℃; for 48h; | 100% |
-
-
56750-58-4
methyl 6-deoxy-2,3-bis-O-(phenylmethyl)-α-D-glucopyranoside

-
-
623-68-7
trans-crotonic anhydride

-
-
250687-53-7
methyl 2,3-di-O-benzyl-4-O-crotonyl-6-deoxy-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 20.5h; | 99% |
-
-
623-68-7
trans-crotonic anhydride

-
-
55697-50-2
methyl 2,4,6-tri-O-benzyl-α-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 120h; | 98% |
-
-
623-68-7
trans-crotonic anhydride

-
-
54522-57-5
methyl-2,3-di-O-benzyl-6-O-(4-toluenesulfonyl)-α-D-glucopyranoside

-
-
366801-70-9
methyl 2,3-di-O-benzyl-4-O-crotonyl-6-O-p-toluenesulfonyl-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 23h; | 98% |
-
-
623-68-7
trans-crotonic anhydride

-
-
366801-67-4
methyl 2,3-di-O-pivaloyl-6-O-p-toluenesulfonyl-α-D-glucopyranoside

-
-
366801-69-6
methyl 4-O-crotonyl-2,3-di-O-pivaloyl-6-O-p-toluenesulfonyl-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 23h; | 98% |
-
-
623-68-7
trans-crotonic anhydride

-
-
90719-32-7
(S)-4-Benzyl-2-oxazolidinone

-
-
90719-30-5
(4S)-3-((E)-2-butenoyl)-4-benzyl-2-oxazolidinone

| Conditions | Yield |
|---|---|
| With triethylamine; lithium chloride In tetrahydrofuran at -15 - 20℃; for 12h; | 97% |
| With triethylamine; lithium chloride In tetrahydrofuran for 4h; Ambient temperature; | 88% |
-
-
19488-48-3
methyl O-2,3,6-tri-O-benzyl-α-D-glucopyranoside

-
-
623-68-7
trans-crotonic anhydride

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 21h; | 97% |

| Conditions | Yield |
|---|---|
| With ytterbium(III) chloride In tetrahydrofuran | 97% |
-
-
623-68-7
trans-crotonic anhydride

-
-
1352481-42-5
5-(2-((4S,6R)-6-((2S,3S)-3-(benzyloxy)-2-hydroxybutyl)-2,2-diisopropyl-1,3,2-dioxasilinan-4-yl)propan-2-yl)furan-2-carbaldehyde

-
-
1352481-43-6
(E)-((2S,3S)-3-(benzyloxy)-1-((4R,6S)-6-(2-(5-formylfuran-2-yl)propan-2-yl)-2,2-diisopropyl-1,3,2-dioxasilinan-4-yl)butan-2-yl) but-2-enoate

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0℃; for 4h; Inert atmosphere; | 97% |
-
-
942062-83-1
2-[2-((tert-butyldimethylsilanyloxy)methyl)phenyl]propan-1-ol

-
-
623-68-7
trans-crotonic anhydride

-
-
942062-84-2
but-2-enoic acid 2-[2-((tert-butyldimethylsilanyloxy)methyl)phenyl]propyl ester

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0℃; for 4h; | 96% |

| Conditions | Yield |
|---|---|
| Acidic catalyst In cyclohexane Heating; | 95% |
-
-
623-68-7
trans-crotonic anhydride

-
-
707-37-9
1,3-dimethyl-5-adamantanol

-
-
134690-48-5
3,5-Dimethyl-1-adamantyl crotonate

| Conditions | Yield |
|---|---|
| Acidic catalyst In cyclohexane Heating; | 95% |
-
-
623-68-7
trans-crotonic anhydride

-
-
128044-91-7
(1R,2R,3S,4R)-3-O-tert-Butyldimethylsilyl-1,2-O-cyclohexylidene-5-hydroxymethyl-5-cyclohexen-1,2,3,4-tetraol

-
-
128044-92-8
(1R,2R,3S,4R)-3-O-tert-Butyldimethylsilyl-5-crotonyloxymethyl-1,2-O-cyclohexylidene-5-cyclohexen-1,2,3,4-tetraol

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane | 95% |
| With pyridine; dmap In dichloromethane for 12h; Ambient temperature; | 95% |
-
-
623-68-7
trans-crotonic anhydride

-
-
62853-52-5
methyl 6-deoxy-2,3-di-O-methyl-α-D-glucopyranoside

-
-
250687-55-9
methyl 4-O-crotonyl-6-deoxy-2,3-di-O-methyl-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 13h; | 95% |
-
-
623-68-7
trans-crotonic anhydride

-
-
168297-85-6
(S)-4-benzyl-5,5-dimethyloxazolidin-2-one

-
-
618436-90-1
(S)-3-((E)-but-2-enoyl)-4-benzyl-5,5-dimethyloxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine; lithium chloride In tetrahydrofuran at -78 - 20℃; for 12h; | 94% |
-
-
623-68-7
trans-crotonic anhydride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20 - 30℃; for 1h; | 94% |
-
-
623-68-7
trans-crotonic anhydride

-
-
366801-72-1
methyl 6-deoxy-2,3-di-O-pivaloyl-α-D-glucopyranoside

-
-
250687-54-8
methyl 4-O-crotonyl-6-deoxy-2,3-di-O-pivaloyl-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 19h; | 93% |
-
-
53008-65-4
methyl 2,3,4-tri-O-benzyl-D-glucopyranoside

-
-
623-68-7
trans-crotonic anhydride

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 18h; | 92% |
-
-
623-68-7
trans-crotonic anhydride

-
-
184715-41-1
methyl 2,3-di-O-benzyl-6-O-pivaloyl-α-D-glucopyranoside

-
-
250687-57-1
methyl 2,3-di-O-benzyl-4-O-crotonyl-6-O-pivaloyl-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 7h; | 92% |
-
-
497-25-6
dimethylenecyclourethane

-
-
623-68-7
trans-crotonic anhydride

-
-
109299-92-5
3-((E)-but-2-enoyl)oxazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine; lithium chloride In tetrahydrofuran | 92% |
-
-
32981-86-5, 71629-92-0, 92999-93-4, 115509-92-7
10-Deacetylbaccatine III

-
-
623-68-7
trans-crotonic anhydride

| Conditions | Yield |
|---|---|
| With cerium(III) chloride In tetrahydrofuran at 25℃; for 20h; Under N2; | 91% |
| cerium(III) chloride In tetrahydrofuran at 25℃; for 20h; | 91% |
-
-
623-68-7
trans-crotonic anhydride

| Conditions | Yield |
|---|---|
| Stage #1: (E)-N-(4-(3-hydroxyhexa-1,5-dien-1-yl)phenyl)nicotinamide With dmap; triethylamine In dichloromethane at 0℃; Stage #2: trans-crotonic anhydride In dichloromethane at 0 - 20℃; for 3h; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: trimethylsilyl cyanide; benzaldehyde; iron(III) chloride In nitromethane at 0℃; for 0.5h; Stage #2: trans-crotonic anhydride In nitromethane at 20℃; for 3h; | 89% |

-
-
623-68-7
trans-crotonic anhydride

-
-
201230-82-2
carbon monoxide

-
-
615-36-1
2-bromoaniline

-
-
1217821-56-1
(E)-2-(prop-1-en-1-yl)-4H-benzo[d][1,3]oxazin-4-one

| Conditions | Yield |
|---|---|
| With potassium tetrachloropalladate(II); N-ethyl-N,N-diisopropylamine; catacxium A In toluene at 100℃; under 1500.15 Torr; for 16h; Autoclave; | 88% |

-
-
623-68-7
trans-crotonic anhydride


| Conditions | Yield |
|---|---|
| With dmap In pyridine at 20℃; for 5h; | 88% |
-
-
623-68-7
trans-crotonic anhydride

-
-
94594-90-8
(2R)-bornane-10,2-sultam

-
-
94668-55-0
(1S)-N-crotyl-2,10-camphorsultam

| Conditions | Yield |
|---|---|
| With triethylamine; lithium chloride In tetrahydrofuran for 4h; Ambient temperature; | 87% |
-
-
857637-92-4
(4S,5R)-4-benzyl-5-(1'H,1'H,2'H,2'H-perfluorooctyl)-oxazolidin-2-one

-
-
623-68-7
trans-crotonic anhydride

-
-
857637-81-1
(E)-(4S,5R)-4-benzyl-3-(2'-butenoyl)-5-(1'H,1'H,2'H,2'H-perfluorooctyl)-2-oxazolidinone

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; lithium chloride In tetrahydrofuran for 24h; Heating; | 87% |
Crotonic Anhydride Chemical Properties
Chemistry informtion about Crotonic Anhydride (CAS NO.623-68-7) is:
IUPAC Name: [(E)-But-2-Enoyl] (E)-But-2-Enoate
Synonyms: 2-Butenoicacid,Anhydride ; Anhydridkyselinykrotonove ; Crotonicacidanhydride ; Crotonic Anhydride ; 2-Butenoic Anhydride ; Trans-2-Butenoic Anhydride ; 2-Butensureanhydrid ; Crotonsaeureanhydrid
Product Categories: Pharmaceutical Intermediates
MF: C8H10O3
MW: 154.16
EINECS: 210-807-1
Density: 1.038 g/cm3
Flash Point: 106.1 °C
Boiling Point: 247 °C at 760 mmHg
Vapour Pressure: 0.0263 mmHg at 25°C
Enthalpy of Vaporization: 48.41 kJ/mol
Refractive Index: n20/D 1.473(lit.)
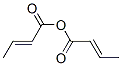
Following is the molecular structure of Crotonic Anhydride (CAS NO.623-68-7) is:
Crotonic Anhydride Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 2830mg/kg (2830mg/kg) | American Industrial Hygiene Association Journal. Vol. 23, Pg. 95, 1962. |
Crotonic Anhydride Consensus Reports
Reported in EPA TSCA Inventory.
Crotonic Anhydride Safety Profile
Moderately toxic by ingestion. A skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes:
 C
C
Risk Statements:
R34:Causes burns.
Safety Statements:
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: UN 3265 8/PG 3
WGK Germany: 3
RTECS: GQ6125000
HazardClass: 8
PackingGroup III
Crotonic Anhydride Specification
To protect yourself, you can put on faceshields, full-face respirator (US), gloves, goggles, multi-purpose combination respirator cartridge (US), type ABEK (EN14387) respirator filter.
Related Products
- Crotonic acid
- Crotonic Acid
- Crotonic Anhydride
- 62369-67-9
- 623-69-8
- 623-70-1
- 623-71-2
- 623-73-4
- 623-76-7
- 62378-68-1
- 623-78-9
- 623-79-0
- 62379-61-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View