-
Name
Cyclododecane
- EINECS 206-033-9
- CAS No. 294-62-2
- Article Data125
- CAS DataBase
- Density 0.791 g/cm3
- Solubility 16μg/L at 20℃
- Melting Point 59-61 °C
- Formula C12H24
- Boiling Point 246.999 °C at 760 mmHg
- Molecular Weight 168.323
- Flash Point 87.585 °C
- Transport Information
- Appearance white solid
- Safety 24/25
- Risk Codes R52/53
-
Molecular Structure
- Hazard Symbols R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment.;
- Synonyms BRN 1901008;
- PSA 0.00000
- LogP 4.68120
Synthetic route

| Conditions | Yield |
|---|---|
| With aluminum oxide; sodium tetrahydroborate; nickel dichloride In hexane at 40℃; Catalytic hydrogenation; | 100% |
| With ethanol; lithium; nickel dichloride; 4,4'-di-tert-butylbiphenyl In tetrahydrofuran at 20℃; for 1h; | 99% |
| With hydrogen; NiCl2-Li-[poly(2-vinyl-naphthalene)-co-(divinylbenzene)] In tetrahydrofuran at 20℃; under 760.051 Torr; for 1h; | 99% |

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel dichloride In tetrahydrofuran; methanol at 0℃; for 0.25h; | 100% |
-
-
41320-42-7
O-dodecyl S-methyl carbonodithioate

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); hypophosphorous acid; sodium hydrogencarbonate In ethanol for 0.5h; Heating; | 100% |
-
-
4904-61-4
cyclododeca-1,5,9-triene

-
A
-
294-62-2
cyclododecane

-
B
-
1501-82-2
cyclododecene

-
C
-
1502-04-1, 67881-13-4
cyclododeca-1,5-diene

| Conditions | Yield |
|---|---|
| With hydrogen at 80 - 160℃; under 760.051 Torr; Reagent/catalyst; Flow reactor; | A 100% B n/a C n/a |
| With hydrogen; Ru4Sn6/Davison 923 mesoporous silica at 119.84℃; under 22502.3 Torr; for 12h; Product distribution; Further Variations:; reaction times; |


| Conditions | Yield |
|---|---|
| With air; trimethylborane; water In benzene at 20℃; | 99% |
| With di-tert-butyl peroxide; Diphenylphosphine oxide In 1,4-dioxane for 16h; Reduction; Heating; | 98% |
| With tris-(trimethylsilyl)silane; 1,1'-azobis(1-cyanocyclohexanenitrile) In water at 100℃; for 4h; | 98% |

| Conditions | Yield |
|---|---|
| With hydrogen at 240℃; under 760.051 Torr; Reagent/catalyst; Flow reactor; | 99% |
| With hydrogen In methanol at 20℃; under 760.051 Torr; for 8h; Reagent/catalyst; | > 99 %Chromat. |
-
-
130534-80-4
Thiocarbonic acid O-cyclododecyl ester O-(4-fluoro-phenyl) ester

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With tris-(trimethylsilyl)silane; triethyl borane; oxygen In benzene at 25℃; for 0.166667h; other reagent: diphenylsilane; | 98% |
| With triethyl borane; diphenylsilane; oxygen In hexane; benzene for 0.166667h; Ambient temperature; | 96% |
| With diphenylsilane; triethyl borane; oxygen In benzene at 25℃; for 0.166667h; Product distribution; Mechanism; same reaction of further thiocarbonyl compounds; other reagents (tris(trimethylsilyl)silane); other temp. and reaction times; | 96% |
-
-
121410-95-5
O-cyclododecyl O-phenyl thionocarbonate

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With 2,2'-azobis-(2,4-dimethylvaleronitrile); tri-n-butyl-tin hydride In tetrahydrofuran at 70℃; for 3h; Solvent; Time; Barton-McCombie Deoxygenation; Inert atmosphere; | 96% |
| With 2,2'-azobis(isobutyronitrile); (2,6-dimethoxy-1-methylcyclohexa-2,5-dienyl)triisopropylsilane In hexane for 15h; Heating; | 92% |
| With 2,2'-azobis(isobutyronitrile); (2,6-dimethoxy-1-methylcyclohexa-2,5-dienyl)triisopropylsilane In hexane for 15h; Heating; | 92% |
-
-
157258-21-4
O-cyclododecyl N-phenyl-thiocarbamate

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); 9,10-dihydro-9,10-dimethyl-9,10-disilaanthracene In benzene for 2h; Heating; | 96% |
| With tris-(trimethylsilyl)silane; 1,1'-azobis(1-cyanocyclohexanenitrile) In water at 100℃; for 4h; | 85% |
| With 2,2'-azobis(isobutyronitrile); tris-(trimethylsilyl)silane In benzene at 80℃; for 0.5h; | 95 % Chromat. |
| With 5,10-dihydro-silanthrene; ABIN In toluene at 80℃; for 2h; | 32 % Chromat. |
| With 2,2'-azobis(isobutyronitrile); tris-(trimethylsilyl)silane In benzene at 80℃; for 0.5h; Mechanism; deoxygenation of various thioxocarbamate der. with variation of reagent and temp.; | 95 % Chromat. |

| Conditions | Yield |
|---|---|
| With naphthalene; water-d2; lithium; nickel dichloride In tetrahydrofuran Ambient temperature; | 93% |

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With 2,2-bis(tert-butylperoxy)butane; tri-tert-butoxysilanethiol In octane for 3h; Heating; | 93% |

| Conditions | Yield |
|---|---|
| With triethylsilane; 2C2H3F3O*BF3 In dichloromethane at 20℃; for 2.5h; | 92% |
| With hydrogenchloride; amalgamated zinc at 155℃; |
-
-
97-94-9
triethyl borane

-
-
4373-12-0
O-cyclododecyl-S-methyl dithiocarbonate

-
A
-
10596-55-1
S-Ethyl S'-methyl dithiocarbonate

-
B
-
294-62-2
cyclododecane

-
C
-
1501-82-2
cyclododecene

-
D
-
88011-88-5
1,1'-bicyclododecane

-
E
-
830-13-7
cyclododecanone

| Conditions | Yield |
|---|---|
| With air In hexane Product distribution; Mechanism; Ambient temperature; variation of temperature; other thiocarbonyl derivatives; | A 91% B 62% C 12% D 9% E 4% |


-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With triethyl borane; HSiPh3 In benzene for 0.166667h; Ambient temperature; | 91% |
-
-
121282-62-0
cyclododecyl isocyanide

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With perhydrodibenzo-18-crown-6; potassium; toluene Ambient temperature; | 90% |

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With tris-(trimethylsilyl)silane; 1,1'-azobis(1-cyanocyclohexanenitrile) In water at 100℃; for 4h; | 90% |
| With 5,10-dihydro-silanthrene; ABIN In toluene at 80℃; for 2h; | 46 % Chromat. |

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With 2,2-bis(tert-butylperoxy)butane; tri-tert-butoxysilanethiol In octane for 3h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; (4-diphenylsilylphenyl)diphenylsilane at 140℃; for 15h; | 89% |
| With di-tert-butyl peroxide; (4-diphenylsilylphenyl)diphenylsilane at 140℃; for 15h; Product distribution; alcohol deoxygenation via acetate; reaction with Ph3SiH; | 89% |
| With di-tert-butyl peroxide; diphenylsilane at 140℃; for 20h; | 75 % Chromat. |
-
-
109275-15-2
2-Cyclododecylsulfanyl-benzothiazole

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With tri-n-butyl-tin hydride; 2,2'-azobis(isobutyronitrile) In benzene for 14h; Heating; | 89% |

| Conditions | Yield |
|---|---|
| With hydrogen; platinum In ethyl acetate at 20℃; for 12h; | 86% |

| Conditions | Yield |
|---|---|
| With magnesium; palladium on activated charcoal In methanol Ambient temperature; | 85% |
| With hydrogen; alkylated polyethyleneimine; palladium In water at 80℃; under 1500.12 Torr; for 5h; |

| Conditions | Yield |
|---|---|
| With water; zinc In acetonitrile at 80℃; for 4h; Sealed tube; Inert atmosphere; | 84% |
| With N,N,N,N,N,N-hexamethylphosphoric triamide; samarium diiodide; isopropyl alcohol In tetrahydrofuran for 0.166667h; Ambient temperature; Yield given; | |
| With zinc; cob(I)alamin In water; acetic acid for 93h; Ambient temperature; Yield given; |
-
-
6221-92-7
cyclododecyl acetate

-
-
789-25-3
HSiPh3

-
A
-
294-62-2
cyclododecane

-
B
-
1929-33-5
triphenylsilyl acetate

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide at 140℃; for 12h; | A 82% B n/a |

-
-
943-93-1
1,2-epoxy-5,9-cyclododecadiene

-
A
-
294-62-2
cyclododecane

-
B
-
1724-39-6
cyclododecanol

-
C
-
830-13-7
cyclododecanone

-
D
-
286-99-7
1,2-epoxycyclododecane

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on silica gel at 160 - 175℃; under 2205.22 - 6615.66 Torr; for 2 - 5h; | A 0.2% B 0.9% C 81.6% D 10.6% |
| With hydrogen; Pd/silica-alumina at 160℃; under 6615.66 Torr; for 2h; | A 0.3% B 2.4% C 70.7% D 25.5% |
| With hydrogen; 5 wt percent Pd/zeolite at 160℃; under 6615.66 Torr; for 2h; | A 0.5% B 1.8% C 69.4% D 27.5% |


| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide at 140℃; for 12h; | A 81% B n/a |


-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With diphenylsilane; triethyl borane; oxygen In benzene at 25℃; for 0.166667h; | 81% |

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tris-(trimethylsilyl)silane In benzene at 80℃; | 76% |
-
-
42604-12-6
cyclododecyl methoxymethyl ether

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With 2,2-bis(tert-butylperoxy)butane; tri-tert-butoxysilanethiol In octane for 3h; Heating; | 74% |
-
-
94473-30-0
cyclododecyl p-nitrophenyl selenide

-
-
294-62-2
cyclododecane

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel dichloride In tetrahydrofuran; methanol at 0℃; for 0.25h; | 71% |

| Conditions | Yield |
|---|---|
| With hydrogen at 240℃; under 760.051 Torr; Reagent/catalyst; Flow reactor; | A 71% B 26% |
-
-
294-62-2
cyclododecane

-
-
34039-83-3
chlorocyclododecane

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; benzophenone In acetonitrile at 27℃; for 24h; Reagent/catalyst; Irradiation; regioselective reaction; | 95% |
| With tetrachloromethane at 250℃; for 7h; Inert atmosphere; Autoclave; | 35% |
| With methanol; tetrachloromethane; molybdenum hexacarbonyl at 170℃; for 1h; Temperature; Time; Sealed tube; |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide In 1,2-dichloro-ethane at 130℃; for 4h; Sealed tube; | 93% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid; dibenzoyl peroxide In 1,2-dichloro-ethane at 100℃; for 4h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With cytochrome P450 enzyme CYP101B1 In aq. buffer pH=7.4; Kinetics; Reagent/catalyst; Enzymatic reaction; | A 92% B n/a |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; Selectfluor In water; acetone at 20℃; for 16h; Inert atmosphere; Irradiation; | 90% |
| With trifluoroacetic acid; dibenzoyl peroxide In 1,2-dichloro-ethane at 100℃; for 4h; Inert atmosphere; | 78% |
-
-
294-62-2
cyclododecane

-
-
38857-88-4
bis(2,2,2-trichlorethyl)azodicarboxylate

-
-
1407999-64-7
C18H28Cl6N2O4

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide In 1,2-dichloro-ethane at 20 - 80℃; for 5h; Inert atmosphere; chemoselective reaction; | 87% |

| Conditions | Yield |
|---|---|
| With 2,2,2-trifluoroethanol; bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane at 20℃; for 17h; Reagent/catalyst; Minisci Aromatic Substitution; Inert atmosphere; Irradiation; Green chemistry; | 86% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-tetramethylpiperidinium triflate In neat liquid at 120℃; for 12h; Reagent/catalyst; Green chemistry; | 85% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; di-tert-butyl peroxide; Bathocuproine; nickel(II) acetate tetrahydrate In fluorobenzene at 140℃; for 24h; Inert atmosphere; | 85% |
| With di-tert-butyl peroxide; Bathocuproine; nickel(II) acetate tetrahydrate at 140℃; for 24h; Inert atmosphere; Schlenk technique; | 82% |
Cyclododecane Specification
The Cyclododecane has the CAS registry number 294-62-2. Its EINECS number is 206-033-9. This chemical's molecular formula is C12H24 and molecular weight is 168.32. What's more, its systematic name is cyclododecane. It is used as compound semiconductors, polymerization catalysts, or in organic synthesis. It is also used as an intermediate in production of flame retardants, detergents, and other chemicals.
Physical properties of Cyclododecane are: (1)ACD/LogP: 6.33; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 6.325; (4)ACD/LogD (pH 7.4): 6.325; (5)ACD/BCF (pH 5.5): 37762.496; (6)ACD/BCF (pH 7.4): 37762.496; (7)ACD/KOC (pH 5.5): 65742.078; (8)ACD/KOC (pH 7.4): 65742.078; (9)Index of Refraction: 1.433; (10)Molar Refractivity: 55.341 cm3; (11)Molar Volume: 212.883 cm3; (12)Polarizability: 21.939×10-24cm3; (13)Surface Tension: 25.952 dyne/cm; (14)Density: 0.791 g/cm3; (15)Flash Point: 87.585 °C; (16)Enthalpy of Vaporization: 46.454 kJ/mol; (17)Boiling Point: 246.999 °C at 760 mmHg; (18)Vapour Pressure: 0.041 mmHg at 25°C.
Preparation of Cyclododecane: this chemical can be prepared by cyclododecene at the temperature of 40 °C. This reaction will need reagents sodium borohydride, moist Al2O3 and solvent hexane. This reaction will also need catalyst NiCl2·6H2O. The yield is about 100%.
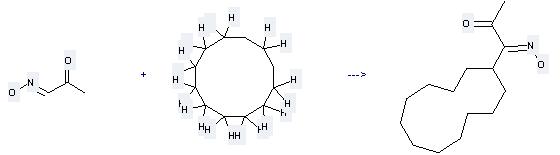
Uses of Cyclododecane: it can be used to produce 1-cyclododecyl-propane-1,2-dione 1-oxime at the temperature of 60 °C. It will need reagent di-t-butyl peroxalate with the reaction time of 12 hours. The yield is about 40%.
You can still convert the following datas into molecular structure:
(1)SMILES: C1CCCCCCCCCCC1
(2)Std. InChI: InChI=1S/C12H24/c1-2-4-6-8-10-12-11-9-7-5-3-1/h1-12H2
(3)Std. InChIKey: DDTBPAQBQHZRDW-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | subcutaneous | > 10gm/kg (10000mg/kg) | Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 20(5/6), Pg. 772, 1973. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View