-
Name
Cycloleucine
- EINECS 200-144-6
- CAS No. 52-52-8
- Article Data18
- CAS DataBase
- Density 1.207 g/cm3
- Solubility 5 g/100 mL in waier
- Melting Point 320 °C (dec.)(lit.)
- Formula C6H11NO2
- Boiling Point 256.1 °C at 760 mmHg
- Molecular Weight 129.159
- Flash Point 108.7 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance white to beige crystalline flakes or powder
- Safety 22-24/25
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  Xn
Xn
- Synonyms 1-Amino-1-carboxycyclopentane;1-Aminocyclopentane-1-carboxylic acid;1-Aminocyclopentanecarboxylic acid;ACPC;Cycloleucin;NSC 1026;NSC 112194;NSC 112195;NSC 112197;WR 14997;
- PSA 63.32000
- LogP 1.04280
Synthetic route
-
-
49830-37-7
1-amino-1-cyanocyclopentane

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In 1,4-dioxane for 8h; Inert atmosphere; Reflux; | 99% |
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With sodium hydroxide Heating; | 75% |
| With barium dihydroxide In water for 3h; Heating; | 71% |
| With barium hydroxide octahydrate; water In water at 160℃; for 2h; Autoclave; | 70% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; palladium Hydrogenation; |

| Conditions | Yield |
|---|---|
| With ethanol; ammonium chloride Verseifen des Nitrils durch Erhitzen mit Salzsaeure; |
-
-
89985-89-7
1-<(Diphenylmethylene)-amino>-1-cyclopentanecarbonitrile

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride 1.) ether, 12 h, room temp., 2.) reflux; Yield given. Multistep reaction; |

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid at 140℃; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 64 percent / (NH4)2CO3 / ethanol; H2O / 2 h / 58 - 60 °C 2: 71 percent / Ba(OH)2*8H2O / H2O / 3 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: H2O; NH2OH+HCl 2: aqueous HCl 3: palladium; aqueous NH3 / Hydrogenation View Scheme | |
| Multi-step reaction with 2 steps 1: ammonium chloride; ammonium hydroxide / isopropyl alcohol / 20 °C / Inert atmosphere 2: hydrogenchloride; water / 1,4-dioxane / 8 h / Inert atmosphere; Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ethanol; H2O / Heating 2: 60percent H2SO4 / 10 h / 140 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 73 percent / ethanol; H2O / 6 h / Heating 2: 75 percent / 3 N aq. NaOH / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aqueous NaHSO3 2: aqueous HCl 3: palladium; aqueous NH3 / Hydrogenation View Scheme |
-
-
99848-19-8
1-hydroxyamino-cyclopentanecarbonitrile

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aqueous HCl 2: palladium; aqueous NH3 / Hydrogenation View Scheme |

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With water; potassium hydroxide In tetrahydrofuran at 20℃; for 6h; | 0.77 g |
-
-
67-56-1
methanol

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
60421-23-0
cycloleucine methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride In methanol at -15 - 20℃; | 100% |
| With thionyl chloride at 20℃; for 3h; Cooling with ice; | 98% |
| With thionyl chloride at 20℃; for 3h; Inert atmosphere; | 98% |
-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
60421-23-0
cycloleucine methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| 100% | |
| In methanol |
-
-
64-18-6
formic acid

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
15026-77-4
1-formamidocyclopentane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With acetic anhydride at 50℃; for 4h; | 98% |
-
-
17356-08-0
thiourea

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
14109-98-9
cyclopentanespiro-5-(2-thiohydantoin) {cyclopentanespiro-5-(2-thioxoimidazolidin-4-one)}

| Conditions | Yield |
|---|---|
| at 225 - 230℃; | 96% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0℃; for 3h; Reflux; | 93.5% |
| With thionyl chloride at 0 - 20℃; Inert atmosphere; | 67% |
| With hydrogenchloride Heating; | |
| With thionyl chloride r.t., 15 h, reflux, 1 h; |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
35264-09-6
1-(tert-butoxycarbonylamino)cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; acetone Inert atmosphere; | 90% |
| With tetramethyl ammoniumhydroxide In acetonitrile for 72h; Ambient temperature; | 88% |
| With sodium hydroxide In tetrahydrofuran; water at 55℃; for 18h; Inert atmosphere; | 85% |
-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
10316-79-7
(1-aminocyclopentyl)methanol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 1h; | 89% |
| With sodium tetrahydroborate; sulfuric acid In tetrahydrofuran; diethyl ether at 20℃; for 15h; | 75% |
| Multi-step reaction with 2 steps 1: thionyl chloride / 0 - 20 °C / Inert atmosphere 2: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 2 h / 0 - 110 °C / Inert atmosphere View Scheme |
-
-
98-88-4
benzoyl chloride

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
6306-10-1
1-benzoylamino-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene at 0 - 10℃; for 2h; | 89% |
| With sodium hydroxide In water at 0 - 20℃; for 5h; |

| Conditions | Yield |
|---|---|
| Stage #1: 1-amino-1-cyclopentanecarboxylic acid; benzyl alcohol With toluene-4-sulfonic acid In toluene for 24h; Heating / reflux; Stage #2: With hydrogenchloride In water; acetone | 88.5% |
-
-
141-75-3
butyryl chloride

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
945267-44-7
1-butyrylamino-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene at 0 - 10℃; for 2h; | 87% |
-
-
64-17-5
ethanol

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
1664-35-3
ethyl 1-aminocyclopentanecarboxylate

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 86.5% |
| With hydrogenchloride Destillieren des Hydrochlorids nach Zusatz von Bleihydroxyd unter vermindertem Druck; | |
| With hydrogenchloride Heating; | |
| Stage #1: ethanol; 1-amino-1-cyclopentanecarboxylic acid With sulfuric acid at 0℃; for 24h; Heating / reflux; Stage #2: With sodium hydrogencarbonate In water |
-
-
79-03-8
propionyl chloride

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
945267-43-6
1-propionylamino-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene at 0 - 10℃; for 2h; | 85% |
-
-
39827-11-7
benzothiophene-2-carboxylic acid chloride

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1-amino-1-cyclopentanecarboxylic acid With N,O-bis-(trimethylsilyl)-acetamide In dichloromethane for 1.5h; Stage #2: benzothiophene-2-carboxylic acid chloride In dichloromethane at 20℃; Further stages.; | 85% |
| Stage #1: 1-amino-1-cyclopentanecarboxylic acid With N,O-bis-(trimethylsilyl)-acetamide In dichloromethane at 20℃; Inert atmosphere; Stage #2: benzothiophene-2-carboxylic acid chloride In dichloromethane at 20℃; Inert atmosphere; |
-
-
142-61-0
Hexanoyl chloride

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
945267-45-8
1-hexanoylamino-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene at 0 - 10℃; for 2h; | 84% |
-
-
64-17-5
ethanol

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
22649-37-2
1-amino-cyclopentanecarboxylic acid ethyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride for 4h; Heating; | 83% |
| With hydrogenchloride at 23℃; for 48h; | 6.57 g |
-
-
638-29-9
n-valeryl chloride

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
15026-80-9
N-pentanoylaminocyclopentane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene at 0 - 10℃; for 2h; | 82% |
| Stage #1: n-valeryl chloride; 1-amino-1-cyclopentanecarboxylic acid With sodium hydroxide In water; toluene at 0 - 10℃; for 2 - 3h; Stage #2: With hydrogenchloride In water for 0.25h; pH=2.0 - 2.5; |
-
-
133690-92-3
[(2'-cyanobiphenyl-4-yl)methyl]amine

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1-amino-1-cyclopentanecarboxylic acid With phosphorus pentachloride In chloroform at 55 - 60℃; for 1h; Stage #2: [(2'-cyanobiphenyl-4-yl)methyl]amine In chloroform at 60℃; Further stages.; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-amino-1-cyclopentanecarboxylic acid With chloro-trimethyl-silane; N,O-bis-(trimethylsilyl)-acetamide In dichloromethane for 1h; Stage #2: 2-Nitrobenzenesulfonyl chloride In dichloromethane Further stages.; | 81% |
-
-
501-53-1
benzyl chloroformate

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
17191-44-5
1-{[(benzyloxy)carbonyl]amino}cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tert-butyl methyl ether; water at 23℃; for 3h; pH=10.5 - 11.5; Inert atmosphere; | 80% |
| Stage #1: benzyl chloroformate; 1-amino-1-cyclopentanecarboxylic acid With sodium carbonate In 1,4-dioxane; water at 20℃; Stage #2: With lithium hydroxide In tetrahydrofuran; water at 20℃; Stage #3: In tetrahydrofuran; diethyl ether pH=2; Acidic conditions; | 78% |
| Stage #1: benzyl chloroformate; 1-amino-1-cyclopentanecarboxylic acid With sodium carbonate In 1,4-dioxane; water at 20℃; Stage #2: With hydrogenchloride In 1,4-dioxane; water pH=2; | 78% |
-
-
613-84-3
2-hydroxy-5-methylbenzaldehyde

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
929602-66-4
1-[(2-hydroxy-5-methyl-benzyl)amino]cyclopentane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| 76% |
-
-
50-00-0
formaldehyd

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
933690-12-1
1-(dimethylamino)cyclopentane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With formic acid In water at 80℃; for 2.5h; | 75% |
-
-
638-29-9
n-valeryl chloride

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
15026-81-0
2-butyl-3-oxa-1-aza-spiro[4.4]non-1-en-4-one

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 80℃; for 5h; | 74.4% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; water | 74% |
-
-
3642-06-6
Bicyclo<4.4.0>deca-3,8-dien-1,6-dicarbonsaeureanhydrid

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
78388-62-2
1-(11,13-Dioxo-12-aza-tricyclo[4.4.3.01,6]trideca-3,8-dien-12-yl)-cyclopentanecarboxylic acid

| Conditions | Yield |
|---|---|
| With pyridine In neat (no solvent) at 250℃; | 73% |
-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
635-93-8
5-chlorosalicyclaldehyde

-
-
929602-63-1
1-[(2-hydroxy-5-chloro-benzyl)amino]cyclopentane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| 73% |
-
-
366-18-7
[2,2]bipyridinyl

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
1628897-39-1
[CuII(bpy)(ACPC)]ClO4·H2O

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; for 1h; | 73% |


| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; for 1h; Inert atmosphere; | 73% |

-
-
941-69-5
N-phenyl-maleimide

-
-
65-22-5
pyridoxal hydrochloride

-
-
52-52-8
1-amino-1-cyclopentanecarboxylic acid

-
-
117088-34-3
4'-(3-hydroxy-5-hydroxymethyl-2-methylpyridin-4-yl)-6',8'-dioxo-7'-phenylspiro(cyclopentane-1,2'-<3',7'>diazabicyclo<3.3.0>octane)

| Conditions | Yield |
|---|---|
| With sodium acetate In water; acetonitrile for 3h; Heating; | 70% |

Cycloleucine Consensus Reports
Cycloleucine Specification
The IUPAC name of Cycloleucine is 1-aminocyclopentane-1-carboxylic acid. With the CAS registry number 52-52-8, it is also named as Cyclopentanecarboxylic acid, 1-amino-, L-. The product's categories are Pharmacetical; Amino Acids; Amino Acids; Amino Acids & Derivatives; Cycloalkanes, and the other registry number is 15313-85-6. Besides, it is white to beige crystalline flakes or powder, which should be stored in sealed, cool and dry place at room temperature. In addition, its molecular formula is C6H11NO2 and molecular weight is 129.16.
The other characteristics of this product can be summarized as: (1)EINECS: 200-144-6; (2)ACD/LogP: -0.05; (3)# of Rule of 5 Violations: 0; (4)ACD/LogD (pH 5.5): -2.55; (5)ACD/LogD (pH 7.4): -2.55; (6)ACD/BCF (pH 5.5): 1; (7)ACD/BCF (pH 7.4): 1; (8)ACD/KOC (pH 5.5): 1; (9)ACD/KOC (pH 7.4): 1; (10)#H bond acceptors: 3; (11)#H bond donors: 3; (12)#Freely Rotating Bonds: 2; (13)Index of Refraction: 1.522; (14)Molar Refractivity: 32.62 cm3; (15)Molar Volume: 106.9 cm3; (16)Surface Tension: 53.2 dyne/cm; (17)Density: 1.207 g/cm3; (18)Flash Point: 108.7 °C; (19)Melting point: 320 °C; (20)Water solubility: 5 g/100 mL; (21)Enthalpy of Vaporization: 54.35 kJ/mol; (22)Boiling Point: 256.1 °C at 760 mmHg; (23)Vapour Pressure: 0.00481 mmHg at 25 °C.
Preparation of Cycloleucine: this chemical can be prepared by 1,3-Diaza-spiro[4.4]nonane-2,4-dione.
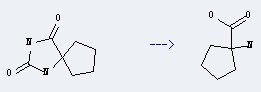
This reaction needs 3 N aq. NaOH by heating. The yield is 75 %.
Uses of Cycloleucine: this chemical is a non-metabolisable amino acid. It is a specific and reversible inhibitor of nucleic acid methylation. It is widely used in biochemical experiments. Additionally, it can react with Ethanol to get 1-Amino-cyclopentanecarboxylic acid ethyl ester.
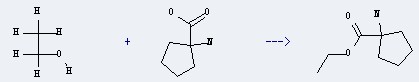
This reaction needs HCl. The yield is 86.5 %.
When you are using this chemical, please be cautious about it as the following: it is harmful if swallowed. Please do not breathe dust. And you should avoid contact with skin and eyes.
People can use the following data to convert to the molecule structure.
(1)SMILES: O=C(O)C1(N)CCCC1
(2)InChI: InChI=1/C6H11NO2/c7-6(5(8)9)3-1-2-4-6/h1-4,7H2,(H,8,9)
(3)InChIKey: NILQLFBWTXNUOE-UHFFFAOYAN
(4)Std. InChI: InChI=1S/C6H11NO2/c7-6(5(8)9)3-1-2-4-6/h1-4,7H2,(H,8,9)
(5)Std. InChIKey: NILQLFBWTXNUOE-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | intravenous | 4gm/kg (4000mg/kg) | Toxicology and Applied Pharmacology. Vol. 18, Pg. 469, 1971. | |
| dog | LD50 | intravenous | 300mg/kg (300mg/kg) | Journal of Medicinal and Pharmaceutical Chemistry. Vol. 3, Pg. 1, 1961. | |
| dog | LD50 | oral | 300mg/kg (300mg/kg) | Journal of Medicinal and Pharmaceutical Chemistry. Vol. 3, Pg. 1, 1961. | |
| guinea pig | LD50 | oral | 140mg/kg (140mg/kg) | Journal of Medicinal and Pharmaceutical Chemistry. Vol. 3, Pg. 1, 1961. | |
| human | TDLo | oral | 60mg/kg (60mg/kg) | BEHAVIORAL: ANOREXIA (HUMAN GASTROINTESTINAL: NAUSEA OR VOMITING | Journal of Medicinal and Pharmaceutical Chemistry. Vol. 3, Pg. 1, 1961. |
| mouse | LD50 | intraperitoneal | 119mg/kg (119mg/kg) | National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, | |
| mouse | LD50 | oral | 309mg/kg (309mg/kg) | Journal of Medicinal and Pharmaceutical Chemistry. Vol. 3, Pg. 1, 1961. | |
| mouse | LD50 | subcutaneous | 375mg/kg (375mg/kg) | National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, | |
| quail | LD50 | oral | > 316mg/kg (316mg/kg) | Ecotoxicology and Environmental Safety. Vol. 6, Pg. 149, 1982. | |
| rat | LD50 | intravenous | 340mg/kg (340mg/kg) | Journal of Medicinal and Pharmaceutical Chemistry. Vol. 3, Pg. 1, 1961. | |
| rat | LD50 | oral | 290mg/kg (290mg/kg) | Journal of Medicinal and Pharmaceutical Chemistry. Vol. 3, Pg. 1, 1961. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View