-
Name
3,3-Dimethyl-D(-)-cysteine
- EINECS 200-148-8
- CAS No. 52-67-5
- Article Data44
- CAS DataBase
- Density 1.205 g/cm3
- Solubility 11.1 g/100 mL (20 ºC)
- Melting Point 210 °C (dec.)(lit.)
- Formula C5H11NO2S
- Boiling Point 251.772 °C at 760 mmHg
- Molecular Weight 149.214
- Flash Point 106.068 °C
- Transport Information
- Appearance white powder
- Safety 26-36-24/25-22
- Risk Codes 36/37/38-40-20/21/22
-
Molecular Structure
- Hazard Symbols Xi,T,Xn
- Synonyms Valine,3-mercapto-, D- (8CI);(-)-Penicillamine;(2S)-2-Amino-3-methyl-3-sulfanylbutanoic acid;(S)-3,3-Dimethylcysteine;(S)-Penicillamine;2-Amino-3-mercapto-3-methylbutanoic acid;Cuprenil;Cuprimine;Cupripen;D-3-Mercaptovaline;D-Mercaptovaline;D-Penamine;D-Penicillamine;DPA;Depamine;Depen;Kuprenil;Mercaptyl;Metalcaptase;NSC 81549;Pendramine;Penicillamin;Penicillamine;Perdolat;Reduced D-penicillamine;Reduced penicillamine;Sufirtan;Trolovol;b-Thiovaline;H-D-Pen-OH;
- PSA 102.12000
- LogP 0.80700
Synthetic route
-
-
113-98-4, 21794-94-5, 68244-03-1
benzylpenicillin potassium salt

-
-
106-49-0
p-toluidine

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
124827-15-2
(Z)-N-(p-methylphenyl)-3-(p-methylanilino)-2-(2-phenylacetamido)acrylamide

| Conditions | Yield |
|---|---|
| With acetic acid In water; toluene for 5h; Ambient temperature; Heating; | A 71% B 95% |

-
-
137-07-5
2-amino-benzenethiol

-
-
73184-06-2
(5R,3S)-benzyl-D-penilloic acid

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

| Conditions | Yield |
|---|---|
| With acetic acid In water 1.) reflux, 2 h, 2.) room temperature, 1 h; | A 86% B 93% |

-
-
479-27-6
naphthalene-1,8-diamine

-
-
73184-06-2
(5R,3S)-benzyl-D-penilloic acid

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

| Conditions | Yield |
|---|---|
| With acetic acid In water for 2h; Heating; | A 93% B 87% |

-
-
73184-06-2
(5R,3S)-benzyl-D-penilloic acid

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

| Conditions | Yield |
|---|---|
| With acetic acid; naphthalene-1,8-diamine In water for 2h; Heating; | A 93% B 87% |
-
-
121766-89-0
benzylpenicilloic acid α-phenylethylamide

-
A
-
51-17-2
benzoimidazole

-
B
-
52-67-5
3,3-dimethyl-D-cysteine

-
C
-
102016-26-2
phenaceturic acid α-phenethylamide

| Conditions | Yield |
|---|---|
| With 1,2-diamino-benzene In water; acetic acid for 1.5h; Heating; | A 59% B 87% C 91% |

-
-
121766-89-0
benzylpenicilloic acid α-phenylethylamide

-
-
95-54-5
1,2-diamino-benzene

-
A
-
51-17-2
benzoimidazole

-
B
-
52-67-5
3,3-dimethyl-D-cysteine

-
C
-
102016-26-2
phenaceturic acid α-phenethylamide

| Conditions | Yield |
|---|---|
| In water; acetic acid for 1.5h; Heating; | A 59% B 87% C 91% |

-
-
121766-89-0
benzylpenicilloic acid α-phenylethylamide

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
121766-91-4, 121842-57-7
N-phenethyl-α-phenylacetamido-2-benzothiazolidineacetamide

| Conditions | Yield |
|---|---|
| With 2-amino-benzenethiol In water; acetic acid for 2h; Heating; | A 83% B 89% |
-
-
121766-89-0
benzylpenicilloic acid α-phenylethylamide

-
-
137-07-5
2-amino-benzenethiol

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
121766-91-4, 121842-57-7
N-phenethyl-α-phenylacetamido-2-benzothiazolidineacetamide

| Conditions | Yield |
|---|---|
| In water; acetic acid for 2h; Heating; | A 83% B 89% |

-
-
121766-90-3
benzylpenicilloic acid α-anilide

-
A
-
51-17-2
benzoimidazole

-
B
-
52-67-5
3,3-dimethyl-D-cysteine

-
C
-
15440-35-4
N-phenyl-N2-(phenylacetyl)glycinamide

| Conditions | Yield |
|---|---|
| With 1,2-diamino-benzene In water; acetic acid for 1.5h; Heating; | A 58% B 86% C 81% |

-
-
137-07-5
2-amino-benzenethiol

-
-
66317-00-8
<2R-<2α(R*),4β>>-5,5-dimethyl-2-<2-oxo-1-<(phenylacetyl)amino>-2-<(phenylmethyl)amino>ethyl>-4-thiazolidinecarboxylic acid

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
121766-92-5, 121767-02-0
N-Benzyl-2-(2,3-dihydro-benzothiazol-2-yl)-2-phenylacetylamino-acetamide

| Conditions | Yield |
|---|---|
| In water; acetic acid for 2h; Heating; | A 81% B 86% |

-
-
66317-00-8
<2R-<2α(R*),4β>>-5,5-dimethyl-2-<2-oxo-1-<(phenylacetyl)amino>-2-<(phenylmethyl)amino>ethyl>-4-thiazolidinecarboxylic acid

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
121766-92-5, 121767-02-0
N-Benzyl-2-(2,3-dihydro-benzothiazol-2-yl)-2-phenylacetylamino-acetamide

| Conditions | Yield |
|---|---|
| With 2-amino-benzenethiol In water; acetic acid for 2h; Heating; | A 81% B 86% |
-
-
66317-00-8
<2R-<2α(R*),4β>>-5,5-dimethyl-2-<2-oxo-1-<(phenylacetyl)amino>-2-<(phenylmethyl)amino>ethyl>-4-thiazolidinecarboxylic acid

-
A
-
51-17-2
benzoimidazole

-
B
-
52-67-5
3,3-dimethyl-D-cysteine

-
C
-
15440-34-3
N-benzyl-2-(2-phenylacetamido)acetamide

| Conditions | Yield |
|---|---|
| With 1,2-diamino-benzene In water; acetic acid for 1.5h; Heating; | A 57% B 80% C 85% |

-
-
121766-89-0
benzylpenicilloic acid α-phenylethylamide

-
-
479-27-6
naphthalene-1,8-diamine

-
A
-
204-02-4
1-H-perimidine

-
B
-
52-67-5
3,3-dimethyl-D-cysteine

-
C
-
102016-26-2
phenaceturic acid α-phenethylamide

| Conditions | Yield |
|---|---|
| In water; acetic acid for 2h; Heating; | A 85% B 69% C 74% |


-
-
52-67-5
3,3-dimethyl-D-cysteine

| Conditions | Yield |
|---|---|
| Stage #1: (S)-2-tert-butoxyamide-3-methyl-3-thioacetoxybutyric acid With hydrogenchloride In 1,4-dioxane; water at 0℃; Reflux; Stage #2: With pyridine In ethanol | 81% |
-
-
66317-01-9
<2R-<2α(R*),4β>>-2-<2-(ethylamino)-2-oxo-1-<(phenylacetyl)amino>ethyl>-5,5-dimethyl-4-thiazolidinecarboxylic acid

-
A
-
51-17-2
benzoimidazole

-
B
-
52-67-5
3,3-dimethyl-D-cysteine

-
C
-
25443-71-4
N-phenylacetyl-glycine ethylamide

| Conditions | Yield |
|---|---|
| With 1,2-diamino-benzene In water; acetic acid for 1.5h; Heating; | A 55% B 71% C 79% |

-
-
66317-01-9
<2R-<2α(R*),4β>>-2-<2-(ethylamino)-2-oxo-1-<(phenylacetyl)amino>ethyl>-5,5-dimethyl-4-thiazolidinecarboxylic acid

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
121766-94-7, 121767-04-2
2-(2,3-Dihydro-benzothiazol-2-yl)-N-ethyl-2-phenylacetylamino-acetamide

| Conditions | Yield |
|---|---|
| With 2-amino-benzenethiol In water; acetic acid for 2h; Heating; | A 74% B 79% |
-
-
66317-01-9
<2R-<2α(R*),4β>>-2-<2-(ethylamino)-2-oxo-1-<(phenylacetyl)amino>ethyl>-5,5-dimethyl-4-thiazolidinecarboxylic acid

-
-
137-07-5
2-amino-benzenethiol

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
121766-94-7, 121767-04-2
2-(2,3-Dihydro-benzothiazol-2-yl)-N-ethyl-2-phenylacetylamino-acetamide

| Conditions | Yield |
|---|---|
| In water; acetic acid for 2h; Heating; | A 74% B 79% |


| Conditions | Yield |
|---|---|
| With acetic acid In water; toluene for 4h; Heating; | A 79% B 9% C 7% |

-
-
150-61-8
N,N'-diphenylethylenediamine

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
55055-34-0
N-formyl-N,N'-diphenylethylenediamine

-
C
-
102016-26-2
phenaceturic acid α-phenethylamide

| Conditions | Yield |
|---|---|
| In acetic acid Heating; | A 51% B 63% C 76% |
| In acetic acid for 5h; Mechanism; Heating; var. penicilloic acid α-amides; | A 51% B 63% C 76% |


-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
102016-26-2
phenaceturic acid α-phenethylamide

| Conditions | Yield |
|---|---|
| With N,N'-diphenylethylenediamine In acetic acid for 5h; Heating; | A 51% B 76% |

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
15440-35-4
N-phenyl-N2-(phenylacetyl)glycinamide

| Conditions | Yield |
|---|---|
| With N,N'-diphenylethylenediamine In acetic acid Heating; | A 49% B 75% |

| Conditions | Yield |
|---|---|
| With acetic acid In methanol; water | 73.2% |

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
15440-34-3
N-benzyl-2-(2-phenylacetamido)acetamide

| Conditions | Yield |
|---|---|
| With N,N'-diphenylethylenediamine In acetic acid Heating; | A 47% B 72% |
-
-
104-94-9
4-methoxy-aniline

-
-
121766-89-0
benzylpenicilloic acid α-phenylethylamide

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

| Conditions | Yield |
|---|---|
| In water; acetic acid; toluene Heating; | A 71% B n/a |

-
-
121766-90-3
benzylpenicilloic acid α-anilide

-
-
137-07-5
2-amino-benzenethiol

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
121766-93-6, 121767-03-1
2-(2,3-Dihydro-benzothiazol-2-yl)-N-phenyl-2-phenylacetylamino-acetamide

| Conditions | Yield |
|---|---|
| In water; acetic acid for 2h; Heating; | A 71% B 68% |

-
-
121766-90-3
benzylpenicilloic acid α-anilide

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
121766-93-6, 121767-03-1
2-(2,3-Dihydro-benzothiazol-2-yl)-N-phenyl-2-phenylacetylamino-acetamide

| Conditions | Yield |
|---|---|
| With 2-amino-benzenethiol In water; acetic acid for 2h; Heating; | A 71% B 68% |

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

-
B
-
25443-71-4
N-phenylacetyl-glycine ethylamide

| Conditions | Yield |
|---|---|
| With N,N'-diphenylethylenediamine In acetic acid Heating; | A 42% B 71% |

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

| Conditions | Yield |
|---|---|
| With N,N'-diphenylethylenediamine In acetic acid Heating; | A 47% B 69% |
-
-
106-49-0
p-toluidine

-
-
121766-89-0
benzylpenicilloic acid α-phenylethylamide

-
A
-
52-67-5
3,3-dimethyl-D-cysteine

| Conditions | Yield |
|---|---|
| In water; acetic acid; toluene Heating; | A 68% B n/a |


| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane for 168h; Ambient temperature; | 100% |
-
-
52-67-5
3,3-dimethyl-D-cysteine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
6482-24-2
2-Bromoethyl methyl ether

-
-
856417-53-3
(2S)-2-((tert-butoxycarbonyl)amino)-3-((2-methoxyethyl)thio)-3-methylbutanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3,3-dimethyl-D-cysteine; di-tert-butyl dicarbonate; 2-Bromoethyl methyl ether With sodium hydroxide; ethanol at 0 - 20℃; for 15h; Stage #2: di-tert-butyl dicarbonate With sodium hydroxide; water at 20℃; for 15h; | 100% |


| Conditions | Yield |
|---|---|
| With sodium hydride In methanol at 20℃; Inert atmosphere; | 99% |
| With sodium hydride In methanol at 20℃; Inert atmosphere; | 99% |
-
-
67-56-1
methanol

-
-
52-67-5
3,3-dimethyl-D-cysteine

-
-
34297-27-3
D-penicillamine methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 20℃; | 98% |
| With hydrogenchloride at 20℃; | 98% |
| With thionyl chloride at 0 - 20℃; for 48.5h; | 91% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethyl acetate; tert-butyl alcohol | 98% |
| With sodium hydroxide In tert-butyl alcohol at 20℃; |
-
-
72428-60-5
1,3-bis(diphenylphosphino)propane digold(I) dichloride

-
-
64-17-5
ethanol

-
-
52-67-5
3,3-dimethyl-D-cysteine

-
-
7732-18-5
water

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 20℃; for 2h; | 97% |

-
-
50-00-0
formaldehyd

-
-
52-67-5
3,3-dimethyl-D-cysteine

-
-
22916-26-3
(S)-5,5-dimethyl-1,3-thiazolidine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In ethanol; water at 20℃; for 18h; | 96% |
| In water at 0 - 20℃; for 23h; | 81% |
| With pyridine In ethanol; water at 110℃; for 0.0833333h; Microwave irradiation; | 76% |

-
-
52-67-5
3,3-dimethyl-D-cysteine

| Conditions | Yield |
|---|---|
| In water stirring of BiOCl into a soln. of D-(-)-penicillamine in water at 40°C; stirring for 15 min at 40°C; filtration;; crystallization on storing at room temperature; washing with cold water; drying; elem. anal.;; | 96% |

| Conditions | Yield |
|---|---|
| With [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate; triethyl phosphite In aq. phosphate buffer; acetonitrile at 20℃; for 0.5h; pH=6.5; Irradiation; | 96% |

-
-
52-67-5
3,3-dimethyl-D-cysteine

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: acetylacetone; N2-atmosphere; dropwise addn. of Au-complex soln. to 2 equiv. of ligand soln. (room temp.), stirring (1 h); filtration (Celite), concn., pptn. on Et2O addn., filtration, recrystn. (CH2Cl2/Et2O), drying (vac.); elem. anal.; | 95% |

| Conditions | Yield |
|---|---|
| With KOH In potassium hydroxide aq. KOH; AgNO3 soln. (pH 5) added to alkaline soln. of penicillamine; solid filtered, washed (EtOH), dried (P4O10); elem. anal.; | 95% |


| Conditions | Yield |
|---|---|
| With KOH; HCl In water aurate soln. (pH 5) added to alkaline soln. of penicillamine, pH 9, thenHCl added dropwise, final pH 4; solid filtered, washed (EtOH), dried (P4O10); elem. anal.; | 95% |


| Conditions | Yield |
|---|---|
| Stage #1: 3,3-dimethyl-D-cysteine; tetrahydrothiophene gold(I)chloride; bis-diphenylphosphinomethane In ethanol; dichloromethane at 20℃; for 1h; Darkness; Stage #2: With sodium hydroxide In ethanol; dichloromethane Stage #3: water In methanol at 20℃; for 840h; | 95% |


| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 20℃; for 2h; | 95% |


| Conditions | Yield |
|---|---|
| In methanol; water for 4h; Ambient temperature; | 93% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydrogencarbonate In water at 70℃; for 0.75h; | 91.8% |
-
-
52-67-5
3,3-dimethyl-D-cysteine

-
-
67-64-1
acetone

-
-
72744-87-7
(4S)-2,2,5,5-tetramethylthiazolidine-4-carboxylic acid

| Conditions | Yield |
|---|---|
| at 55℃; for 2h; | 91.2% |
| for 2h; Condensation; Heating; | 90% |
| In water | 88% |
| In water for 15h; Ambient temperature; | 70% |
-
-
52-67-5
3,3-dimethyl-D-cysteine

-
-
519-05-1
opianic acid

-
-
97272-80-5
(3S,10R)-2,3,5,10-Tetrahydro-6,7-dimethoxy-2,2-dimethyl-5-oxo-<1,3>-thiazolo<2,3-a>isoindol-3-carbonsaeure

| Conditions | Yield |
|---|---|
| In water for 1h; Heating; | 91% |

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethylguanidine In tetrahydrofuran at 50℃; for 1h; Inert atmosphere; | 91% |
-
-
52-67-5
3,3-dimethyl-D-cysteine

-
-
37128-99-7
N-methyl2-piperidone diethyl acetal

-
-
105099-13-6, 122861-60-3
(S)-3-Mercapto-3-methyl-2-[1-methyl-piperidin-(2E)-ylideneamino]-butyric acid

| Conditions | Yield |
|---|---|
| In ethanol at 20 - 25℃; for 5h; | 90% |

| Conditions | Yield |
|---|---|
| In phosphate buffer; water at 20℃; for 0.666667h; pH=7.4; | 90% |
| In phosphate buffer at 37℃; pH=7.4; Product distribution; Kinetics; |

| Conditions | Yield |
|---|---|
| In benzene | A 90% B n/a |

-
-
84152-18-1
[(ClAu)]3(1,1,1-tris(diphenylphosphinomethyl)ethane)

-
-
52-67-5
3,3-dimethyl-D-cysteine

-
-
7732-18-5
water

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 1h; | 90% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium carbonate In methanol; water; N,N-dimethyl-formamide | 89.6% |

D-(-)-Penicillamine Specification
The D,3-Mercaptovaline with CAS registry number of 52-67-5 is also called D-Valine, 3-mercapto-. The IUPAC name is (2S)-2-amino-3-methyl-3-sulfanylbutanoic acid. Its EINECS registry number is 200-148-8. In addition, the molecular formula is C5H11NO2S and the molecular weight is 149.21. It is a kind of white to off-white crystalline powder and belongs to the classes of Amino Acids; Biochemistry; non-Proteinorganic Amino Acids; Miscellaneous Compounds; Antibiotics. And it is stable and incompatible with strong oxidizing agents.
Physical properties about this chemical are: (1)ACD/LogP: 0.85; (2)# of Rule of 5 Violations: 0 ; (3)ACD/BCF (pH 5.5): 1; (4)ACD/BCF (pH 7.4): 1; (5)ACD/KOC (pH 5.5): 1; (6)ACD/KOC (pH 7.4): 1; (7)#H bond acceptors: 3; (8)#H bond donors: 3; (9)#Freely Rotating Bonds: 4; (10)Polar Surface Area: 102.12 Å2; (11)Index of Refraction: 1.528; (12)Molar Refractivity: 38.134 cm3; (13)Molar Volume: 123.845 cm3; (14)Polarizability: 15.117 ×10-24cm3; (15)Surface Tension: 48.155 dyne/cm; (16)Density: 1.205 g/cm3; (17)Flash Point: 106.068 °C; (18)Enthalpy of Vaporization: 53.854 kJ/mol; (19)Boiling Point: 251.772 °C at 760 mmHg; (20)Vapour Pressure: 0.006 mmHg at 25°C.
Preparation of D,3-Mercaptovaline: it can be prepared by penicillin through synthesis or degradation. It also can be prepared by penicillin G potassium by means of hydrolysis.
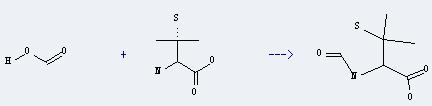
Uses of D,3-Mercaptovaline: it can be used as antidoteit of some heavy metals such as copper and mercury. And it can be used in the treatment of Wilson's disease and rheumatoid arthritis. It does not apply to the hematopoiesis function obstacle patients, kidney function decline patients and who allergic to penicillin. In addition, it can react with formic acid to get N-formyl-penicillamine. This reaction will need reagent acetic anhydride. The reaction time is 3 hours at ambient temperature. The yield is about 78%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin and harmful by inhalation, in contact with skin and if swallowed. The evidence of a carcinogenic effect is limited. During using it, do not breathe dust and wear suitable protective clothing. Moreover, avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: CC(C)([C@H](C(=O)O)N)S
(2)InChI: InChI=1/C5H11NO2S/c1-5(2,9)3(6)4(7)8/h3,9H,6H2,1-2H3,(H,7,8)/t3-/m0/s1
(3)InChIKey: VVNCNSJFMMFHPL-VKHMYHEABH
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | oral | 40mg/kg/1W-I (40mg/kg) | BLOOD: LEUKOPENIA SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Archives of Internal Medicine. Vol. 145, Pg. 2271, 1985. |
| human | TDLo | oral | 21mg/kg/D (21mg/kg) | KIDNEY, URETER, AND BLADDER: PROTEINURIS BLOOD: LEUKOPENIA BLOOD: THROMBOCYTOPENIA | JAMA, Journal of the American Medical Association. Vol. 240, Pg. 1870, 1978. |
| human | TDLo | oral | 893mg/kg/30W- (893mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA MUSCULOSKELETAL: OTHER CHANGES | Journal of Rheumatology. Vol. 11, Pg. 251, 1984. |
| man | TDLo | oral | 400mg/kg/4W-I (400mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BLOOD: HEMORRHAGE | Journal of Rheumatology. Vol. 13, Pg. 963, 1986. |
| man | TDLo | oral | 482mg/kg/19W- (482mg/kg) | BLOOD: THROMBOCYTOPENIA SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Archives of Internal Medicine. Vol. 143, Pg. 1487, 1983. |
| man | TDLo | unreported | 3200mg/kg/84W (3200mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | British Medical Journal. Vol. 296, Pg. 1332, 1988. |
| mouse | LD50 | intraperitoneal | 298mg/kg (298mg/kg) | Yakugaku Zasshi. Journal of Pharmacy. Vol. 94, Pg. 1419, 1974. | |
| mouse | LD50 | intravenous | 3840mg/kg (3840mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Arzneimittel-Forschung. Drug Research. Vol. 25, Pg. 162, 1975. |
| mouse | LD50 | oral | 720mg/kg (720mg/kg) | Pharmaceutical Chemistry Journal Vol. 21, Pg. 842, 1987. | |
| mouse | LD50 | subcutaneous | 3810mg/kg (3810mg/kg) | Drugs in Japan Vol. 6, Pg. 758, 1982. | |
| rat | LD50 | intraperitoneal | 2080mg/kg (2080mg/kg) | Drugs in Japan Vol. 6, Pg. 758, 1982. | |
| rat | LD50 | intravenous | 2gm/kg (2000mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 1434, 1972. | |
| rat | LD50 | oral | 6170mg/kg (6170mg/kg) | Drugs in Japan Vol. 6, Pg. 758, 1982. | |
| rat | LD50 | subcutaneous | 4020mg/kg (4020mg/kg) | Drugs in Japan Vol. 6, Pg. 758, 1982. | |
| women | LDLo | oral | 150mg/kg/30D- (150mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS CARDIAC: ARRHYTHMIAS (INCLUDING CHANGES IN CONDUCTION) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Annals of Internal Medicine. Vol. 98, Pg. 327, 1983. |
| women | TDLo | oral | 105mg/kg/6W-I (105mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | British Medical Journal. Vol. 294, Pg. 1101, 1987. |
| women | TDLo | oral | 112mg/kg/1W-I (112mg/kg) | BLOOD: AGRANULOCYTOSIS SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Archives of Internal Medicine. Vol. 145, Pg. 2271, 1985. |
| women | TDLo | oral | 900mg/kg/26W- (900mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Journal of Rheumatology. Vol. 12, Pg. 583, 1985. |
| women | TDLo | oral | 650gm/kg/81W- (650000mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS MUSCULOSKELETAL: OTHER CHANGES | Arthritis and Rheumatism. Vol. 29, Pg. 560, 1986. |
Related Products
- D-(-)-Penicillamine
- 526-75-0
- 5267-64-1
- 52677-44-8
- 52678-29-2
- 526-78-3
- 52679-49-9
- 52682-86-7
- 526-83-0
- 52683-82-6
- 52685-39-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View