-
Name
distamycin A hydrochloride from*streptomyces dist
- EINECS 229-505-6
- CAS No. 6576-51-8
- Article Data3
- CAS DataBase
- Density
- Solubility CLEAR YELLOW TO GREEN SOLUTION AT 20MG/ML IN ETHANOL
- Melting Point 184-187° (Arcamone, 1967). Also reported as 189-193° from ethanol-ethyl acetate (Bialer)
- Formula C22H27 N9 O4 . Cl H
- Boiling Point 715.4 °C at 760 mmHg
- Molecular Weight 517.975
- Flash Point 386.4 °C
- Transport Information
- Appearance YELLOW TO YELLOW WITH A BROWN CAST POWDER
- Safety Poison by intraperitoneal and intravenous routes. Mutation data reported. When heated to decomposition it emits very toxic fumes of NOx and HCl.
- Risk Codes 43
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1H-Pyrrole-2-carboxamide,N-[5-[[(3-amino-3-iminopropyl)amino]carbonyl]-1-methyl-1H-pyrrol-3-yl]-4-[[[4-(formylamino)-1-methyl-1H-pyrrol-2-yl]carbonyl]amino]-1-methyl-,monohydrochloride (9CI); N,4':N',4''-Ter[pyrrole-2-carboxamide],N''-(2-amidinoethyl)-4-formamido-1,1',1''-trimethyl-, hydrochloride (7CI);N,4':N',4''-Ter[pyrrole-2-carboxamide],N''-(2-amidinoethyl)-4-formamido-1,1',1''-trimethyl-, monohydrochloride (8CI);Distamycin A hydrochloride; Distamycin hydrochloride; MEN 10399; Stallimycinhydrochloride
- PSA 181.06000
- LogP 3.67870
Synthetic route
-
-
77727-43-6
N-Succinimidyl 4-<<<4-<<<4-(Formylamino)-1-methylpyrrol-2-yl>carbonyl>amino>-1-methylpyrrol-2-yl>carbonyl>amino>-1-methylpyrrole-2-carboxylate

-
-
77152-88-6
(1-amino-3-ammoniopropylidene)ammonium dihydrobromide

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydrogencarbonate In 1,4-dioxane; water 1.) 15 min, ice-cooled, 2.) room temperature, overnight; Yield given; |
-
-
3786-88-7
1-methyl-4-<1-methyl-4-(1-methyl-4-aminopyrrole-2-carboxamido)pyrrole-2-carboxamido>pyrrole-2-carboxamidopropionamidine hydrochloride

-
-
3197-61-3
N-formylimidazole

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; methanol at -40℃; for 0.5h; Yield given; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 43 percent / 1.) 70percent nitric acid, acetic anhydride / 1.) a) -15 deg C, 0.5 h, b) RT, 20 min 2: SOCl2 / tetrahydrofuran / 0.08 h / Heating 3: Hunig's base / tetrahydrofuran / -20 - 20 °C 4: 1.) H2, 2.) HCl / 10percent Pd/C / 1.) methanol, 40 deg C, 1 atm, 2.) isopropanol, -30 deg C 5: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 6: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 7: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 8: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme | |
| Multi-step reaction with 8 steps 1: 43 percent / 1.) 70percent nitric acid, acetic anhydride / 1.) a) -15 deg C, 0.5 h, b) RT, 20 min 2: SOCl2 / tetrahydrofuran / 0.08 h / Heating 3: 1.) H2, 2.) Hunig's base / 10percent Pd/C / 1.) MeOH, 1 atm, 2.) THF, 3) THF, RT, 30 min 4: 95 percent / NaOH / H2O; ethanol / Heating 5: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 6: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 7: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 8: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme |
-
-
13138-78-8
1-methyl-4-nitro-pyrrol-2-carboxylic acid

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: SOCl2 / tetrahydrofuran / 0.08 h / Heating 2: Hunig's base / tetrahydrofuran / -20 - 20 °C 3: 1.) H2, 2.) HCl / 10percent Pd/C / 1.) methanol, 40 deg C, 1 atm, 2.) isopropanol, -30 deg C 4: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 5: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 6: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 7: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme | |
| Multi-step reaction with 7 steps 1: SOCl2 / tetrahydrofuran / 0.08 h / Heating 2: 1.) H2, 2.) Hunig's base / 10percent Pd/C / 1.) MeOH, 1 atm, 2.) THF, 3) THF, RT, 30 min 3: 95 percent / NaOH / H2O; ethanol / Heating 4: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 5: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 6: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 7: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme | |
| Multi-step reaction with 8 steps 1: H2, 1.0 M Na2CO3(aq.) / 5percent Pd/C / 3.5 h / 3102.9 Torr / Ambient temperature 2: tetrahydrofuran; diethyl ether / 1.) 1 h, ice cooled, 2.) 2 h, ambient temperature 3: 1-ethyl-3-<3-(dimethylamino)propyl>carbodiimide hydrochloride / dimethylformamide / 40 °C 4: TFA / CH2Cl2 5: EDC / dimethylformamide / 40 °C 6: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 7: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 8: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
97950-76-0
1-methyl-4-(1-methyl-4-nitropyrrole-2-carboxamido)pyrrole-2-carboxylic acid

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 2: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 3: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 4: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme |
-
-
69910-20-9
methyl 1-methyl-4-(1-methyl-4-nitropyrrole-2-carboxamido)pyrrole-2-carbonate

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 95 percent / NaOH / H2O; ethanol / Heating 2: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 3: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 4: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 5: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme |
-
-
28494-51-1
1-methyl-4-nitro-1H-pyrrole-2-carbonyl chloride

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: Hunig's base / tetrahydrofuran / -20 - 20 °C 2: 1.) H2, 2.) HCl / 10percent Pd/C / 1.) methanol, 40 deg C, 1 atm, 2.) isopropanol, -30 deg C 3: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 4: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 5: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 6: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme | |
| Multi-step reaction with 6 steps 1: 1.) H2, 2.) Hunig's base / 10percent Pd/C / 1.) MeOH, 1 atm, 2.) THF, 3) THF, RT, 30 min 2: 95 percent / NaOH / H2O; ethanol / Heating 3: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 4: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 5: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 6: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme |
-
-
13138-76-6
1-methyl-4-nitropyrrole-2-carboxylic acid methyl ester

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 92 percent / aq. NaOH / Heating 2: SOCl2 / tetrahydrofuran / 0.08 h / Heating 3: Hunig's base / tetrahydrofuran / -20 - 20 °C 4: 1.) H2, 2.) HCl / 10percent Pd/C / 1.) methanol, 40 deg C, 1 atm, 2.) isopropanol, -30 deg C 5: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 6: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 7: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 8: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme | |
| Multi-step reaction with 8 steps 1: 92 percent / aq. NaOH / Heating 2: SOCl2 / tetrahydrofuran / 0.08 h / Heating 3: 1.) H2, 2.) Hunig's base / 10percent Pd/C / 1.) MeOH, 1 atm, 2.) THF, 3) THF, RT, 30 min 4: 95 percent / NaOH / H2O; ethanol / Heating 5: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 6: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 7: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 8: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme |
-
-
3185-95-3
1-methyl-4-nitro-1H-pyrrole-2-carboxylic acid (2-cyanoethyl)amide

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 1.) H2, 2.) HCl / 10percent Pd/C / 1.) methanol, 40 deg C, 1 atm, 2.) isopropanol, -30 deg C 2: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 3: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 4: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 5: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme |
-
-
2522-28-3
3-<1-methyl-4-<1-methyl-4-(1-methyl-4-nitropyrrole-2-carboxamido)pyrrole-2-carboxamido>pyrrole-2-carboxamido>propionitrile

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 2: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 3: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme |
-
-
6576-50-7
N-(3-amino-3-iminopropyl)-1-methyl-4-{[(1-methyl-4-{[(1-methyl-4-nitro-1H-pyrrol-2-yl)carbonyl]amino}-1H-pyrrol-2-yl)carbonyl]amino}-1H-pyrrole-2-carboxamide hydrochloride

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 2: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme |

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 64 percent / 1,8-bis(dimethylamino)naphthalene, dicyclohexylcarbodiimide / tetrahydrofuran; dimethylformamide / 3 h / Ambient temperature 2: 1.) HCl, 2.) NH3 / 1.) EtOH, RT, 1.5 h, 2.) EtOH, 1 h, RT 3: 70 percent / H2 / 10percent Pd/C / methanol / 40 °C 4: methanol; tetrahydrofuran / 0.5 h / -40 °C View Scheme |
-
-
45776-13-4
4-amino-1-methylpyrrole-2-carboxylic acid

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: tetrahydrofuran; diethyl ether / 1.) 1 h, ice cooled, 2.) 2 h, ambient temperature 2: 1-ethyl-3-<3-(dimethylamino)propyl>carbodiimide hydrochloride / dimethylformamide / 40 °C 3: TFA / CH2Cl2 4: EDC / dimethylformamide / 40 °C 5: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 6: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 7: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 10 steps 1: tetrahydrofuran; diethyl ether / 1.) 1 h, ice cooled, 2.) 2 h, ambient temperature 2: Cs2CO3 / ethanol; H2O 3: dimethylformamide / 40 °C 4: TFA / CH2Cl2 / 1 h / Ambient temperature; stoppered flask 5: 1-ethyl-3-<3-(dimethylamino)propyl>carbodiimide hydrochloride / dimethylformamide / 40 °C 6: TFA / CH2Cl2 7: EDC / dimethylformamide / 40 °C 8: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 9: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 10: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 10 steps 1: tetrahydrofuran; diethyl ether / 1.) 1 h, ice cooled, 2.) 2 h, ambient temperature 2: Cs2CO3 / ethanol; H2O 3: dimethylformamide / 40 °C 4: TFA / CH2Cl2 / 1 h / Ambient temperature; stoppered flask 5: tetramethylguanidine / dimethylsulfoxide / 20 h / 50 °C 6: TFA / CH2Cl2 7: EDC / dimethylformamide / 40 °C 8: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 9: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 10: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 8 steps 1: tetrahydrofuran; diethyl ether / 1.) 1 h, ice cooled, 2.) 2 h, ambient temperature 2: 71 percent / N,N'-dicyclohexylcarbodiimide / dimethylformamide / 20 h / Ambient temperature 3: tetramethylguanidine / dimethylsulfoxide / 20 h / 50 °C 4: TFA / CH2Cl2 5: EDC / dimethylformamide / 40 °C 6: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 7: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 8: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 5 steps 1: 2.5 h / ice-bath 2: EDC / dimethylformamide / 40 °C 3: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 4: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 5: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 69 percent / 2 M NaOH / ethanol / 1.5 h / Heating 2: H2, 1.0 M Na2CO3(aq.) / 5percent Pd/C / 3.5 h / 3102.9 Torr / Ambient temperature 3: tetrahydrofuran; diethyl ether / 1.) 1 h, ice cooled, 2.) 2 h, ambient temperature 4: 1-ethyl-3-<3-(dimethylamino)propyl>carbodiimide hydrochloride / dimethylformamide / 40 °C 5: TFA / CH2Cl2 6: EDC / dimethylformamide / 40 °C 7: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 8: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 9: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 12 steps 1: 69 percent / 2 M NaOH / ethanol / 1.5 h / Heating 2: H2, 1.0 M Na2CO3(aq.) / 5percent Pd/C / 3.5 h / 3102.9 Torr / Ambient temperature 3: tetrahydrofuran; diethyl ether / 1.) 1 h, ice cooled, 2.) 2 h, ambient temperature 4: Cs2CO3 / ethanol; H2O 5: dimethylformamide / 40 °C 6: TFA / CH2Cl2 / 1 h / Ambient temperature; stoppered flask 7: 1-ethyl-3-<3-(dimethylamino)propyl>carbodiimide hydrochloride / dimethylformamide / 40 °C 8: TFA / CH2Cl2 9: EDC / dimethylformamide / 40 °C 10: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 11: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 12: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 12 steps 1: 69 percent / 2 M NaOH / ethanol / 1.5 h / Heating 2: H2, 1.0 M Na2CO3(aq.) / 5percent Pd/C / 3.5 h / 3102.9 Torr / Ambient temperature 3: tetrahydrofuran; diethyl ether / 1.) 1 h, ice cooled, 2.) 2 h, ambient temperature 4: Cs2CO3 / ethanol; H2O 5: dimethylformamide / 40 °C 6: TFA / CH2Cl2 / 1 h / Ambient temperature; stoppered flask 7: tetramethylguanidine / dimethylsulfoxide / 20 h / 50 °C 8: TFA / CH2Cl2 9: EDC / dimethylformamide / 40 °C 10: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 11: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 12: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 10 steps 1: 69 percent / 2 M NaOH / ethanol / 1.5 h / Heating 2: H2, 1.0 M Na2CO3(aq.) / 5percent Pd/C / 3.5 h / 3102.9 Torr / Ambient temperature 3: tetrahydrofuran; diethyl ether / 1.) 1 h, ice cooled, 2.) 2 h, ambient temperature 4: 71 percent / N,N'-dicyclohexylcarbodiimide / dimethylformamide / 20 h / Ambient temperature 5: tetramethylguanidine / dimethylsulfoxide / 20 h / 50 °C 6: TFA / CH2Cl2 7: EDC / dimethylformamide / 40 °C 8: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 9: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 10: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 7 steps 1: 69 percent / 2 M NaOH / ethanol / 1.5 h / Heating 2: H2, 1.0 M Na2CO3(aq.) / 5percent Pd/C / 3.5 h / 3102.9 Torr / Ambient temperature 3: 2.5 h / ice-bath 4: EDC / dimethylformamide / 40 °C 5: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 6: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 7: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
77716-14-4
Benzyl 4-Amino-1-methylpyrrole-2-carboxylate

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 1-ethyl-3-<3-(dimethylamino)propyl>carbodiimide hydrochloride / dimethylformamide / 40 °C 2: TFA / CH2Cl2 3: EDC / dimethylformamide / 40 °C 4: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 5: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 6: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 6 steps 1: tetramethylguanidine / dimethylsulfoxide / 20 h / 50 °C 2: TFA / CH2Cl2 3: EDC / dimethylformamide / 40 °C 4: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 5: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 6: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
77716-19-9
Benzyl 4-<<(4-Amino-1-methylpyrrol-2-yl)carbonyl>amino>-1-methylpyrrole-2-carboxylate

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: EDC / dimethylformamide / 40 °C 2: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 3: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 4: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
77716-18-8
4-formamido-1-methylimidazole-2-carboxylic acid

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: EDC / dimethylformamide / 40 °C 2: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 3: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 4: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
77716-11-1
4-[(tert-butoxycarbonyl)amino]-1-methyl-1H-pyrrole-2-carboxylic acid

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 1-ethyl-3-<3-(dimethylamino)propyl>carbodiimide hydrochloride / dimethylformamide / 40 °C 2: TFA / CH2Cl2 3: EDC / dimethylformamide / 40 °C 4: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 5: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 6: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 9 steps 1: Cs2CO3 / ethanol; H2O 2: dimethylformamide / 40 °C 3: TFA / CH2Cl2 / 1 h / Ambient temperature; stoppered flask 4: 1-ethyl-3-<3-(dimethylamino)propyl>carbodiimide hydrochloride / dimethylformamide / 40 °C 5: TFA / CH2Cl2 6: EDC / dimethylformamide / 40 °C 7: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 8: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 9: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 9 steps 1: Cs2CO3 / ethanol; H2O 2: dimethylformamide / 40 °C 3: TFA / CH2Cl2 / 1 h / Ambient temperature; stoppered flask 4: tetramethylguanidine / dimethylsulfoxide / 20 h / 50 °C 5: TFA / CH2Cl2 6: EDC / dimethylformamide / 40 °C 7: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 8: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 9: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 7 steps 1: 71 percent / N,N'-dicyclohexylcarbodiimide / dimethylformamide / 20 h / Ambient temperature 2: tetramethylguanidine / dimethylsulfoxide / 20 h / 50 °C 3: TFA / CH2Cl2 4: EDC / dimethylformamide / 40 °C 5: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 6: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 7: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
77716-13-3
benzyl 4-<<(tert-butyloxy)carbonyl>amino>-1-methyl-pyrrole-2-carboxylate

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: TFA / CH2Cl2 / 1 h / Ambient temperature; stoppered flask 2: 1-ethyl-3-<3-(dimethylamino)propyl>carbodiimide hydrochloride / dimethylformamide / 40 °C 3: TFA / CH2Cl2 4: EDC / dimethylformamide / 40 °C 5: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 6: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 7: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 7 steps 1: TFA / CH2Cl2 / 1 h / Ambient temperature; stoppered flask 2: tetramethylguanidine / dimethylsulfoxide / 20 h / 50 °C 3: TFA / CH2Cl2 4: EDC / dimethylformamide / 40 °C 5: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 6: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 7: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
77716-16-6
4-tert-butoxycarbonylamino-1-methyl-1H-pyrrole-2-carboxylic acid benzotriazol-1yl ester

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: tetramethylguanidine / dimethylsulfoxide / 20 h / 50 °C 2: TFA / CH2Cl2 3: EDC / dimethylformamide / 40 °C 4: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 5: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 6: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
77716-15-5
benzyl 4-<<<4-<<(tert-butyloxy)carbonyl>amino>-1-methyl-pyrrol-2-yl>carbonyl>amino>-1-methyl-pyrrole-2-carboxylate

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: TFA / CH2Cl2 2: EDC / dimethylformamide / 40 °C 3: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 4: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 5: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
77716-21-3
4-(4-(4-formamido-1-methyl-1H-pyrrole-2-carboxamido)-1-methyl-1H-pyrrole-2-carboxamido)-1-methyl-1H-pyrrole-2-carboxylic acid

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 2: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
77716-20-2
Benzyl 4-<<<4-<<<4-(Formylamino)-1-methylpyrrol-2-yl>carbonyl>amino>-1-methylpyrrol-2-yl>carbonyl>amino>-1-methylpyrrole-2-carboxylate

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 2: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 3: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
77716-12-2
Caesium; 4-tert-butoxycarbonylamino-1-methyl-1H-pyrrole-2-carboxylate

-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: dimethylformamide / 40 °C 2: TFA / CH2Cl2 / 1 h / Ambient temperature; stoppered flask 3: 1-ethyl-3-<3-(dimethylamino)propyl>carbodiimide hydrochloride / dimethylformamide / 40 °C 4: TFA / CH2Cl2 5: EDC / dimethylformamide / 40 °C 6: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 7: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 8: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme | |
| Multi-step reaction with 8 steps 1: dimethylformamide / 40 °C 2: TFA / CH2Cl2 / 1 h / Ambient temperature; stoppered flask 3: tetramethylguanidine / dimethylsulfoxide / 20 h / 50 °C 4: TFA / CH2Cl2 5: EDC / dimethylformamide / 40 °C 6: H2 / 5percent Pd/C / dimethylformamide / 2 h / 3102.9 Torr / Ambient temperature 7: 88 percent / DCC / dimethylformamide / 1.) 70 min, 0 deg C, 2.) 40 deg C, overnight 8: 1.) NaHCO3, 2.) HCl / H2O; dioxane / 1.) 15 min, ice-cooled, 2.) room temperature, overnight View Scheme |
-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol for 24h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 95% |
-
-
420-04-2
CYANAMID

-
-
6576-51-8
distamycin A hydrochloride

-
-
194483-83-5
N-[5-({[5-({[3-amino-3-(cyanoimino)propyl]amino}carbonyl)-1-methyl-1H-pyrrol-3-yl]amino}carbonyl)-1-methyl-1H-pyrrol-3-yl]-4-(formylamino)-1-methyl-1H-pyrrole-2-carboxamide

| Conditions | Yield |
|---|---|
| Stage #1: CYANAMID With sodium hydride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: distamycin A hydrochloride In N,N-dimethyl-formamide for 2h; | 93% |
| Stage #1: CYANAMID With sodium hydride In N,N-dimethyl-formamide for 0.5h; Stage #2: distamycin A hydrochloride In N,N-dimethyl-formamide for 5h; | 92% |
| Stage #1: CYANAMID With sodium hydride In DMF (N,N-dimethyl-formamide) at 20℃; for 0.5h; Stage #2: distamycin A hydrochloride In DMF (N,N-dimethyl-formamide) at 20℃; for 2h; Stage #3: With acetic acid In DMF (N,N-dimethyl-formamide) pH=7; |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 20℃; for 5.5h; | 92% |
-
-
6576-51-8
distamycin A hydrochloride

-
-
71084-59-8
N-(5-{[(5-{[(2-cyanoethyl)amino]carbonyl}-1-methyl-1H-pyrrol-3-yl)amino]carbonyl}-1-methyl-1H-pyrrol-3-yl)-4-(formylamino)-1-methyl-1H-pyrrole-2-carboxamide

| Conditions | Yield |
|---|---|
| With sodium carbonate In N,N-dimethyl-formamide at 70℃; for 3h; | 87% |
| With succinic acid anhydride; sodium carbonate In N,N-dimethyl-formamide at 70℃; for 3h; | 87% |
| With succinic acid anhydride; potassium carbonate In N,N-dimethyl-formamide for 3h; Ambient temperature; | 75% |
-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 60℃; for 4h; | 75% |

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide at 20℃; for 29h; | 73% |

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide at 20℃; for 12h; | 73% |
| In DMF (N,N-dimethyl-formamide) for 8h; |
-
-
6576-51-8
distamycin A hydrochloride

-
-
74-89-5
methylamine

-
A
-
863319-29-3
N-[5-({[5-({[3-amino-3-(N-methylcarbamoyl)ethyl]amino}carbonyl)-1-methyl-1H-pyrrol-3-yl]amino}carbonyl)-1-methyl-1H-pyrrol-3-yl]-4-(formylamino)-1-methyl-1H-pyrrole-2-carboxamide

-
B
-
17165-05-8
N-[5-({[5-({[2-(carbamoyl)ethyl]amino}carbonyl)-1-methyl-1H-pyrrol-3-yl]amino}carbonyl)-1-methyl-1H-pyrrol-3-yl]-4-(formylamino)-1-methyl-1H-pyrrole-2-carboxamide

| Conditions | Yield |
|---|---|
| With water In N,N-dimethyl-formamide at 80℃; for 3h; | A 18% B 57% |

-
-
6576-51-8
distamycin A hydrochloride

-
-
203258-75-7
N-[5-({[5-({[3-amino-3-(hydroxyimino)propyl]amino}carbonyl)-1-methyl-1H-pyrrol-3-yl]amino}carbonyl)-1-methyl-1H-pyrrol-3-yl]-4-(formylamino)-1-methyl-1H-pyrrole-2-carboxamide

| Conditions | Yield |
|---|---|
| With hydroxylamine In N,N-dimethyl-formamide at 70℃; for 2h; | 52% |
| With TEA; hydroxylamine hydrochloride In N,N-dimethyl-formamide at 70℃; for 1h; | 52% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 80℃; for 6h; | 42% |

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide at 80℃; for 3h; | 32% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 72h; | 20% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide Ambient temperature; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide Ambient temperature; |

| Conditions | Yield |
|---|---|
| With base In N,N-dimethyl-formamide at 70℃; Substitution; |
-
-
6576-51-8
distamycin A hydrochloride

-
-
17165-05-8
N-[5-({[5-({[2-(carbamoyl)ethyl]amino}carbonyl)-1-methyl-1H-pyrrol-3-yl]amino}carbonyl)-1-methyl-1H-pyrrol-3-yl]-4-(formylamino)-1-methyl-1H-pyrrole-2-carboxamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In acetonitrile at 70℃; Hydrolysis; |
-
-
6576-51-8
distamycin A hydrochloride

-
-
203258-75-7
C22H27N9O5

| Conditions | Yield |
|---|---|
| With hydroxylamine In N,N-dimethyl-formamide at 70℃; Substitution; |

| Conditions | Yield |
|---|---|
| With base In N,N-dimethyl-formamide at 70℃; Substitution; |

| Conditions | Yield |
|---|---|
| With base In N,N-dimethyl-formamide at 70℃; Substitution; |

| Conditions | Yield |
|---|---|
| With base In N,N-dimethyl-formamide at 70℃; Substitution; |

| Conditions | Yield |
|---|---|
| With base In N,N-dimethyl-formamide at 70℃; Substitution; |
-
-
6576-51-8
distamycin A hydrochloride

-
-
17165-10-5
4-amino-N-(5-{[(5-{[(3-amino-3-iminopropyl)amino]carbonyl}-1-methyl-1H-pyrrol-3-yl)amino]carbonyl}-1-methyl-1H-pyrrol-3-yl)-1-methyl-1H-pyrrole-2-carboxamide dihydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 20℃; for 24h; |
-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 57 percent / H2O / dimethylformamide / 3 h / 80 °C 2: aq. HCl / aq. ethanol / 24 h / 20 °C 3: TEA / dimethylformamide / 3 h / 20 °C View Scheme |
-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 18 percent / H2O / dimethylformamide / 3 h / 80 °C 2: aq. HCl / aq. ethanol / 24 h / 20 °C 3: TEA / dimethylformamide / 3 h / 20 °C View Scheme |
-
-
6576-51-8
distamycin A hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 52 percent / NH2OH*HCl; TEA / dimethylformamide / 1 h / 70 °C 2: aq. HCl / aq. ethanol / 24 h / 20 °C 3: TEA / dimethylformamide / 3 h / 20 °C View Scheme |
DISTAMYCIN A HYDROCHLORIDE Chemical Properties
MW: 517.97
EINECS: 229-505-6
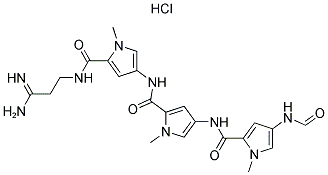
Following is the molecular structure of DISTAMYCIN A HYDROCHLORIDE:
DISTAMYCIN A HYDROCHLORIDE Toxicity Data With Reference
| 1. | dni-mus:lym 1900 nmol/L | CBINA8 Chemico-Biological Interactions. 8 (1974),183. | ||
| 2. | oms-mus:lym 3200 nmol/L | CBINA8 Chemico-Biological Interactions. 8 (1974),183. | ||
| 3. | dnd-mam:lym 200 µmol/ | EJBCAI European Journal of Biochemistry. 26 (1972),81. | ||
| 4. | ipr-rat LD50:169 mg/kg | MDACAP Medicamentos de Actualidad. 13 (1977),319. | ||
| 5. | ipr-mus LD50:160 mg/kg | MDACAP Medicamentos de Actualidad. 13 (1977),319. | ||
| 6. | ivn-mus LD50:75 mg/kg | MEIEDD Merck Index. 11 (1989),1383. |
RTECS :WZ7110000
DISTAMYCIN A HYDROCHLORIDE Safety Profile
Hazard Codes : Xi (Irritant)
Risk Statements: 43 (May cause sensitization by skin contact)
Safety Statements : 36/37 (Wear suitable protective clothing and gloves)
WGK Germany: 3
DISTAMYCIN A HYDROCHLORIDE Specification
Related Products
- Distamycin
- DISTAMYCIN A HYDROCHLORIDE
- Distamycin A/4
- Distamycin A/5
- 65766-32-7
- 65767-22-8
- 6577-41-9
- 65776-63-8
- 6577-89-5
- 6577-95-3
- 6578-06-9
- 65782-04-9
- 657-84-1
- 65792-05-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View