-
Name
Diethyl oxalate
- EINECS 202-464-1
- CAS No. 95-92-1
- Article Data118
- CAS DataBase
- Density 1.086 g/cm3
- Solubility may decompose in water
- Melting Point -41 °C(lit.)
- Formula C6H10O4
- Boiling Point 185.4 °C at 760 mmHg
- Molecular Weight 146.143
- Flash Point 75.6 °C
- Transport Information UN 2525 6.1/PG 3
- Appearance colourless liquid
- Safety 23
- Risk Codes 22-36
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Ethanedioicacid, diethyl ester (9CI);Oxalic acid, diethyl ester (6CI,8CI);Diethylethanedioate;Ethyl oxalate;NSC 8851;Ethanedioicacid, 1,2-diethyl ester;
- PSA 52.60000
- LogP 0.11260
Synthetic route

| Conditions | Yield |
|---|---|
| With enriched hydroxylated graphene oxide In benzene at 120 - 140℃; | 99.3% |
| With sulfuric acid for 1h; Reflux; | 93% |
| With Fe(SO4)3 * xH2O In toluene for 1.2h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With sodium methylate | 95% |

| Conditions | Yield |
|---|---|
| With sodium methylate | 95% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; Oxone In water at 20℃; for 0.166667h; | 95% |

| Conditions | Yield |
|---|---|
| 87% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
64-17-5
ethanol

-
-
98020-30-5
dichloro-(2-chloro-ethoxy)-acetyl chloride

-
A
-
75-00-3
chloroethane

-
B
-
107-06-2
1,2-dichloro-ethane

-
C
-
95-92-1
oxalic acid diethyl ester

| Conditions | Yield |
|---|---|
| zuletzt in der Siedehitze; reagiert analog mit Methanol und Isopropylalkohol; |

-
-
64-17-5
ethanol

-
-
98020-90-7
bis(trichloromethyl) oxalate

-
A
-
541-41-3
chloroformic acid ethyl ester

-
B
-
95-92-1
oxalic acid diethyl ester

-
-
64-17-5
ethanol

-
-
66775-86-8
phenol; compound with oxalic acid

-
A
-
95-92-1
oxalic acid diethyl ester

-
B
-
108-95-2
phenol


| Conditions | Yield |
|---|---|
| im Einschlussrohr; | |
| beim Aufbewahren; |
-
-
617-37-8
oxalic acid monoethyl ester

-
A
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
B
-
95-92-1
oxalic acid diethyl ester

-
C
-
109-94-4
formic acid ethyl ester

| Conditions | Yield |
|---|---|
| bei der Destillation unter gewoehnlichem Druck; |


-
B
-
95-92-1
oxalic acid diethyl ester

| Conditions | Yield |
|---|---|
| beim Erhitzen ueber den Schmelzpunkt; |
-
-
90681-11-1
3-ethoxyoxalylamino-benzoic acid

-
A
-
155049-63-1
3,3'-oxalyldiamino-di-benzoic acid

-
B
-
95-92-1
oxalic acid diethyl ester

| Conditions | Yield |
|---|---|
| at 225℃; |
-
-
110029-67-9
5-acetoxy-6-ethoxyoxalyloxy-2-methyl-benzofuran-3-carboxylic acid ethyl ester

-
A
-
83634-11-1
ethyl 5,6-dihydroxy-2-methyl-1-benzofuran-3-carboxylate

-
B
-
95-92-1
oxalic acid diethyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol |

| Conditions | Yield |
|---|---|
| beim Gluehen; |
-
-
64-17-5
ethanol

-
-
144-62-7
oxalic acid

-
A
-
617-37-8
oxalic acid monoethyl ester

-
B
-
95-92-1
oxalic acid diethyl ester

| Conditions | Yield |
|---|---|
| at 40 - 50℃; bei mehrwoechigem Stehen der gesaettigen Loesung; | |
| With 20 molpercent molybdenum oxide nanoparticle supported on mesoporous silica at 75℃; for 8h; Time; |

-
-
64-17-5
ethanol

-
-
144-62-7
oxalic acid

-
A
-
95-92-1
oxalic acid diethyl ester

-
B
-
109-94-4
formic acid ethyl ester

| Conditions | Yield |
|---|---|
| durch Erhitzen; Nebenprod.2:Kohlensaeurediaethylester; |

-
-
144-62-7
oxalic acid

-
-
621-62-5
chloroacetaldehyde diethyl acetal

-
A
-
107-20-0
2-chloroethanal

-
B
-
95-92-1
oxalic acid diethyl ester

| Conditions | Yield |
|---|---|
| at 100 - 150℃; |


-
-
99-91-2
para-chloroacetophenone

-
-
95-92-1
oxalic acid diethyl ester

-
-
5814-38-0
ethyl 4-chlorobenzoylpyruvate

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 1h; Claisen Condensation; Reflux; | 100% |
| With sodium In ethanol for 16h; Cooling with ice; | 97% |
| With sodium ethanolate In toluene at 50 - 60℃; for 4h; | 90% |
-
-
563-80-4
3-methyl-butan-2-one

-
-
95-92-1
oxalic acid diethyl ester

-
-
64195-85-3
ethyl 2,4-dioxo-5-methylhexanoate

| Conditions | Yield |
|---|---|
| With sodium In ethanol at 0 - 80℃; for 2h; | 100% |
| Stage #1: 3-methyl-butan-2-one; oxalic acid diethyl ester With sodium In ethanol at 0 - 80℃; for 1.75h; Stage #2: With sulfuric acid In ethanol; water at 15 - 25℃; pH=2; | 90% |
| With sodium In ethanol at 20 - 80℃; for 76.25h; Reflux; Inert atmosphere; | 89.7% |
-
-
92-91-1
biphenyl-4-acetaldehyde

-
-
95-92-1
oxalic acid diethyl ester

-
-
41350-17-8
ethyl 3-(4-phenylbenzoyl)-2-ketopropionate

| Conditions | Yield |
|---|---|
| Stage #1: biphenyl-4-acetaldehyde With lithium hexamethyldisilazane In tetrahydrofuran; dichloromethane at -78℃; for 0.75h; Stage #2: oxalic acid diethyl ester In tetrahydrofuran; dichloromethane at 0 - 20℃; for 16h; Stage #3: With water; ammonium chloride In tetrahydrofuran; dichloromethane | 100% |
| With sodium ethanolate In benzene for 4.5h; Ambient temperature; | 70% |
| With sodium | |
| Stage #1: oxalic acid diethyl ester With sodium ethanolate In toluene for 0.166667h; Cooling with ice; Stage #2: biphenyl-4-acetaldehyde In toluene for 4h; Cooling with ice; | |
| With sodium In diethyl ether at 20 - 40℃; |
-
-
95-92-1
oxalic acid diethyl ester

-
-
77-86-1
2-amino-2-hydroxymethyl-1,3-propanediol

-
-
5714-31-8
N,N'-bis[tris(hydroxymethyl)methyl]ethanediamide

| Conditions | Yield |
|---|---|
| at 140℃; for 1h; | 100% |
| In ethylene glycol at 100 - 105℃; for 6h; Conversion of starting material; | 92% |
-
-
95-92-1
oxalic acid diethyl ester

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
35322-20-4
ethyl 3-(4-methoxybenzoyl)pyruvate

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-methoxyphenyl)ethanone With sodium In ethanol at 20℃; for 0.5h; Stage #2: oxalic acid diethyl ester at 20 - 80℃; for 3h; | 100% |
| With sodium hydride In N,N-dimethyl-formamide at 0 - 100℃; | 100% |
| Stage #1: oxalic acid diethyl ester With sodium methylate In diethyl ether at 20℃; Inert atmosphere; Stage #2: 1-(4-methoxyphenyl)ethanone In diethyl ether at 20℃; for 12h; | 92% |
-
-
95-92-1
oxalic acid diethyl ester

-
-
479-27-6
naphthalene-1,8-diamine

-
-
109735-80-0
ethyl 1H-perimidine-2-carboxylate

| Conditions | Yield |
|---|---|
| at 135 - 140℃; for 1.5h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 0 - 20℃; for 20h; Condensation; | 100% |
| Stage #1: acetophenone; oxalic acid diethyl ester With sodium hydride In N,N-dimethyl-formamide at 20 - 50℃; for 0.833333h; Stage #2: With hydrogenchloride In water | 100% |
| With sodium hydride In tetrahydrofuran for 1h; Claisen Condensation; Reflux; | 100% |
-
-
88-15-3
2-Acetylthiophene

-
-
95-92-1
oxalic acid diethyl ester

-
-
36983-36-5
ethyl 2,4-dioxo-4-(thiophen-2-yl)butanoate

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 0 - 20℃; for 20h; Condensation; | 100% |
| With sodium methylate for 0.333333h; | 82% |
| With sodium methylate for 0.333333h; Heating; | 82% |
-
-
3528-17-4
2,3-dihydro-4H-[1]benzothiopyran-4-one

-
-
95-92-1
oxalic acid diethyl ester

-
-
56876-58-5
3-ethoxalyl-2,3-dihydro-1-benzothiopyran-4-one

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 40℃; for 2h; | 100% |
| With sodium In ethanol Claisen Condensation; |

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In diethyl ether at -78 - 20℃; | 100% |
| Stage #1: para-chloroacetophenone With lithium hexamethyldisilazane In tetrahydrofuran; tert-butyl methyl ether at -75 - -70℃; for 1h; Stage #2: oxalic acid diethyl ester at -70 - 20℃; for 19h; | 94% |
| Stage #1: para-chloroacetophenone With lithium hexamethyldisilazane In tetrahydrofuran; diethyl ether at -78℃; for 0.666667h; Stage #2: oxalic acid diethyl ester In tetrahydrofuran; diethyl ether at -78 - 20℃; | 92% |
| With lithium hexamethyldisilazane 1.) ether, -78 deg C, 30 min, 2.) room temperature, 2 h; Yield given. Multistep reaction; |
-
-
61535-21-5
1-(benzyloxy)-3-methyl-2-nitrobenzene

-
-
95-92-1
oxalic acid diethyl ester

-
-
96564-49-7
ethyl 3-(3-benzyloxy-2-nitrophenyl)-pyruvate, potassium salt

| Conditions | Yield |
|---|---|
| With potassium ethoxide In diethyl ether for 20h; Heating; | 100% |
| With potassium tert-butylate In tetrahydrofuran; tert-butyl methyl ether at 20℃; Heating / reflux; | 74% |
| With potassium tert-butylate In tetrahydrofuran; diethyl ether at 20℃; Heating / reflux; |
-
-
3761-92-0
n-hexylmagnesium bromide

-
-
95-92-1
oxalic acid diethyl ester

-
-
67873-26-1
ethyl 2-oxooctanoate

| Conditions | Yield |
|---|---|
| Stage #1: n-hexylmagnesium bromide; oxalic acid diethyl ester In tetrahydrofuran; diethyl ether at -58 - 0℃; for 0.5h; Stage #2: With sulfuric acid; water In tetrahydrofuran; diethyl ether | 100% |
| In tetrahydrofuran; diethyl ether at -60℃; | 88% |
| In tetrahydrofuran at -10℃; for 1h; | 43% |
| In diethyl ether at -78℃; for 1.66667h; Inert atmosphere; | |
| In tetrahydrofuran at -78 - 20℃; for 1h; Inert atmosphere; |
-
-
13291-18-4
isopropenylmagnesium bromide

-
-
95-92-1
oxalic acid diethyl ester

-
-
50331-71-0
ethyl 3-methyl-2-oxobut-3-enoate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether at -70℃; for 0.333333h; | 100% |
| In tetrahydrofuran; diethyl ether at -60℃; | 87% |
| In tetrahydrofuran; diethyl ether at -78℃; for 1.83333h; Inert atmosphere; | 80% |
| In tetrahydrofuran; diethyl ether at -80℃; | 3.75 g |
| In tetrahydrofuran; diethyl ether at -78℃; |
-
-
76179-40-3
2-amino-4,5-difluoroaniline

-
-
95-92-1
oxalic acid diethyl ester

-
-
91895-29-3
6,7-difluoro-1,4-dihydro-2,3-quinoxalinedione

| Conditions | Yield |
|---|---|
| for 16h; Heating; | 100% |
-
-
95-92-1
oxalic acid diethyl ester

-
-
23356-96-9
(S)-1-Pyrrolidin-2-yl-methanol

-
-
85351-62-8
1,2-Bis-((S)-2-hydroxymethyl-pyrrolidin-1-yl)-ethane-1,2-dione

| Conditions | Yield |
|---|---|
| 0 degC-room temp, 2 h, room temp; | 100% |
-
-
6285-05-8
4'-chloropropiophenone

-
-
95-92-1
oxalic acid diethyl ester

-
-
169544-41-6
4-(4-chlorophenyl)-3-methyl-2,4-dioxobutyric acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium In ethanol at 20℃; for 18h; Claisen Condensation; | 100% |
| With lithium tert-butoxide In tetrahydrofuran at 0 - 20℃; for 4h; Claisen Condensation; Inert atmosphere; | 82% |
| Stage #1: oxalic acid diethyl ester With sodium ethanolate In ethanol at 20℃; for 0.166667h; Inert atmosphere; Stage #2: 4'-chloropropiophenone In ethanol at 20℃; for 24h; | 76.9% |
-
-
95-92-1
oxalic acid diethyl ester

-
-
156740-88-4
[5-(4-amino-2,6-dimethyl-phenoxy)-2-hydroxy-phenyl]-(4-fluoro-phenyl)-methanone

-
-
156740-56-6
ethyl N-<4-<3-(4-fluorobenzoyl)-4-hydroxyphenoxy>-3,5-dimethylphenyl>oxamate

| Conditions | Yield |
|---|---|
| at 100℃; for 1h; | 100% |
| In n-heptane |
-
-
109-55-7
1-amino-3-(dimethylamino)propane

-
-
95-92-1
oxalic acid diethyl ester

-
-
25148-90-7
N,N'-bis(3-dimethylaminopropyl)oxalamide

| Conditions | Yield |
|---|---|
| In ethanol Inert atmosphere; Flow reactor; | 100% |
| In tetrahydrofuran at 65℃; for 24h; | 90% |
-
-
99-90-1
para-bromoacetophenone

-
-
95-92-1
oxalic acid diethyl ester

-
-
40155-54-2
ethyl 4-(4-bromophenyl)-2,4-dioxobutanoate

| Conditions | Yield |
|---|---|
| Stage #1: para-bromoacetophenone With sodium In ethanol at 0 - 10℃; for 1h; Stage #2: oxalic acid diethyl ester In ethanol for 1.5h; | 100% |
| With sodium ethanolate In ethanol at 20℃; for 12h; | 85% |
| Stage #1: oxalic acid diethyl ester With sodium ethanolate In benzene at 0℃; Inert atmosphere; Stage #2: para-bromoacetophenone In benzene at 0 - 20℃; for 14.5h; Inert atmosphere; | 75% |
-
-
66067-44-5
(3-acetylphenyl)(phenyl)methanone

-
-
95-92-1
oxalic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride In toluene at 60℃; | 100% |
-
-
95-92-1
oxalic acid diethyl ester

-
-
70334-60-0
3-(azidophenyl) methyl ketone

| Conditions | Yield |
|---|---|
| With sodium hydride In toluene at 60℃; | 100% |
-
-
95-92-1
oxalic acid diethyl ester

-
-
682360-15-2
1-[3-(4-benzoyl-benzyloxy)-phenyl]-ethanone

| Conditions | Yield |
|---|---|
| With sodium hydride In toluene at 60℃; | 100% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate In tetrahydrofuran at 25℃; for 0.00277778h; microwave irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate In tetrahydrofuran at 25℃; for 0.00277778h; microwave irradiation; | 100% |
| With potassium tert-butylate In tetrahydrofuran at 20℃; Claisen Condensation; | 88% |

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In diethyl ether at -78 - 20℃; | 100% |
-
-
95-92-1
oxalic acid diethyl ester

-
-
169192-93-2
2-bromo-6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-one

-
-
870679-54-2
ethyl γ-(7-bromo-1-oxo-2,3,4,5-tetrahydrobenzocyclohepten-2-yl)-α-oxoacetate

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 20℃; Inert atmosphere; | 100% |
| With sodium ethanolate In ethanol at 20℃; for 9h; | 90% |
-
-
95-92-1
oxalic acid diethyl ester

-
-
67-64-1
acetone

-
-
53120-38-0
2-hydroxy-4-oxopent-2-enoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: oxalic acid diethyl ester; acetone With sodium ethanolate In ethanol at 20℃; for 2h; Stage #2: With sulfuric acid In water at 0 - 20℃; | 100% |
| With potassium tert-butylate In tetrahydrofuran at 0 - 20℃; for 16h; | 47% |
| With sodium ethanolate In ethanol at 20℃; for 2h; aldol condensation; |
-
-
883107-05-9
3,6-diacetyl-4(1H)-quinolinone

-
-
95-92-1
oxalic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium ethanolate In tetrahydrofuran at 20℃; for 2h; Claisen condensation; | 100% |

-
-
95-92-1
oxalic acid diethyl ester

-
-
951630-17-4
6-(2-methoxy-phenyl)-2,3-dioxo-1,2,3,4-tetrahydro-quinoxaline-5-carbonitrile

| Conditions | Yield |
|---|---|
| for 5h; Heating / reflux; | 100% |
-
-
947589-65-3
3-acetyl-5-methoxy-1-(4-(trifluoromethyl)benzyl)-1H-indole

-
-
95-92-1
oxalic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium methylate In tetrahydrofuran at 50℃; under 15514.9 Torr; for 0.0666667h; microwave irradiation; | 100% |
Diethyl oxalate Consensus Reports
Diethyl oxalate Specification
The Diethyl oxalate, with the CAS registry number 95-92-1, is also known as Ethanedioicacid, 1,2-diethyl ester. It belongs to the product categories of Pharmaceutical Intermediates; Organics; Analytical Chemistry; Solvents for HPLC & Spectrophotometry; Solvents for Spectrophotometry; C6 to C7; Carbonyl Compounds; Esters; Esters Chromatography; Alpha Sort; Chemical Class; DAlphabetic; DID - DIN Analytical Standards; Food & Beverage Standards; Organic Acids; Volatiles/ Semivolatiles. Its EINECS number is 202-464-1. This chemical's molecular formula is C6H10O4 and molecular weight is 146.14. What's more, its systematic name is diethyl ethanedioate. Its classification code is Skin / Eye Irritant. It is mainly used as an intermediate for phenobarbital, azathioprine, sulfadoxine-pyrimethamine and so on in the pharmaceutical industry. It is also used as a plastic promoter, dyes intermediates and as solvent of cellulose esters, spices. It should be sealed and stored in a cool, ventilated and dry place. Moreover, it should be protected from oxides, acids, bases, heat and fire.
Physical properties of Diethyl oxalate are: (1)ACD/LogP: 0.73; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.73; (4)ACD/LogD (pH 7.4): 0.73; (5)ACD/BCF (pH 5.5): 2.1; (6)ACD/BCF (pH 7.4): 2.1; (7)ACD/KOC (pH 5.5): 59.17; (8)ACD/KOC (pH 7.4): 59.17; (9)#H bond acceptors: 4; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 5; (12)Polar Surface Area: 52.6 Å2; (13)Index of Refraction: 1.41; (14)Molar Refractivity: 33.39 cm3; (15)Molar Volume: 134.5 cm3; (16)Polarizability: 13.23×10-24cm3; (17)Surface Tension: 32.1 dyne/cm; (18)Density: 1.086 g/cm3; (19)Flash Point: 75.6 °C; (20)Enthalpy of Vaporization: 42.16 kJ/mol; (21)Boiling Point: 185.4 °C at 760 mmHg; (22)Vapour Pressure: 0.698 mmHg at 25°C.
Preparation: this chemical can be prepared by oxalic acid and ethanol by heating. This reaction will need reagent Fe(SO4)3 ·xH2O and solvent toluene with the reaction time of 1.2 hours. The yield is about 96%.
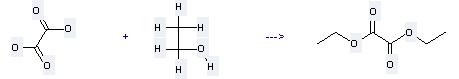
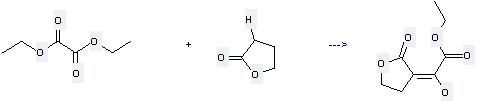
Uses of Diethyl oxalate: it can be used to produce oxo-(2-oxo-tetrahydro-furan-3-yl)-acetic acid ethyl ester at the temperature of 0 - 20 °C. It will need reagent sodium ethoxide and solvent ethanol. The yield is about 96%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful if swallowed. It is irritating to the eyes. You should not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer).
You can still convert the following datas into molecular structure:
(1)InChI: InChI=1S/C6H10O4/c1-3-9-5(7)6(8)10-4-2/h3-4H2,1-2H3
(2)InChIKey: WYACBZDAHNBPPB-UHFFFAOYSA-N
(3)Canonical SMILES: CCOC(=O)C(=O)OCC
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 2gm/kg (2000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ANTIPSYCHOTIC | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 46(5), Pg. 87, 1981. |
Related Products
- Diethyl (1-phenylpropyl)malonate
- Diethyl (2-(cyclohexylamino)vinyl)phosphonate
- Diethyl (2-(triethoxysilyl)ethyl)phosphonate
- Diethyl (2,4,6-trifluorophenyl)malonate
- Diethyl (2-oxopropyl)phosphonate
- Diethyl (2-thienylmethyl)phosphonate
- Diethyl (4-cyanobenzyl)phosphonate
- Diethyl (4-nitrobenzyl)phosphonate
- Diethyl (E)-2,3-diethylbut-2-enedioate
- Diethyl (hydroxymethyl)phosphonate
- 959-22-8
- 95923-44-7
- 959236-11-4
- 959236-14-7
- 959236-54-5
- 959236-59-0
- 959236-97-6
- 959237-20-8
- 959237-31-1
- 959237-34-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View