-
Name
Diflubenzuron
- EINECS 252-529-3
- CAS No. 35367-38-5
- Article Data57
- CAS DataBase
- Density 1.471 g/cm3
- Solubility 0.008 g/100 mL
- Melting Point 230-232 °C
- Formula C14H9ClF2N2O2
- Boiling Point
- Molecular Weight 310.687
- Flash Point
- Transport Information UN 3077
- Appearance colorless to yellow crystals
- Safety 60-61
- Risk Codes 50/53
-
Molecular Structure
-
Hazard Symbols
 C,
C, N
N
- Synonyms 1-(4-chlorophenyl)-3-(2,6-ifluorobenzoyl)urea;Difluron;Larvakil;Benzamide,N-[[(4-chlorophenyl)amino]- carbonyl]-2,6-difluoro-;Micromite (Uniroyal);Dimilin;
- PSA 58.20000
- LogP 4.04400
Synthetic route
-
-
106-47-8
4-chloro-aniline

-
-
148931-18-4
S-allyl N-(2,6-difluorobenzoyl)monothiocarbamate

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| With triethylamine In benzene for 2h; Heating; | 70% |
-
-
106-47-8
4-chloro-aniline

-
-
119448-98-5
N-(Bis-methylsulfanyl-methylene)-2,6-difluoro-benzamide

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| With acetic acid for 6h; Heating; | 69% |
-
-
18063-02-0
2,6-difluorobenzoylchloride

-
-
140-38-5
N-(4-chlorophenyl)urea

-
A
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

-
B
-
122987-01-3
N-(4-chlorophenyl)-2,6-difluorobenzamide

| Conditions | Yield |
|---|---|
| In toluene for 7h; Heating; | A 67% B 5 g |
| In toluene for 7h; Heating; | A 5 g B 5 g |


-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| With 9-(2-mesityl)-10-methylacridinium perchlorate In chloroform at 35℃; for 4h; Irradiation; | 64% |
-
-
60731-73-9
2,6-difluorobenzoyl isocyanate

-
A
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane at 20℃; for 3h; | A n/a B 60.1% |

-
-
60731-73-9
2,6-difluorobenzoyl isocyanate

-
-
18437-66-6
N-(t-butoxycarbonyl)-4-chloroaniline

-
A
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane Heating; | A n/a B 59% |

-
-
64248-56-2
1-bromo-2,6-difluorobenzene

-
-
201230-82-2
carbon monoxide

-
-
140-38-5
N-(4-chlorophenyl)urea

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| With palladium diacetate; triethylamine; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane at 100℃; under 3361.55 Torr; for 4h; Microwave irradiation; | 45% |

-
-
30379-59-0
benzyl N-methylcarbamate

-
-
60731-73-9
2,6-difluorobenzoyl isocyanate

-
-
106-47-8
4-chloro-aniline

-
A
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Stage #1: benzyl N-methylcarbamate With sulfur dichloride Stage #2: 4-chloro-aniline With pyridine In tetrahydrofuran at 20℃; Stage #3: 2,6-difluorobenzoyl isocyanate In 1,2-dichloro-ethane at 20℃; | A n/a B 11.9% |

-
-
18063-03-1
2,6-difluorobenzamide

-
-
104-12-1
p-chlorphenylisocyanate

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| In toluene for 5h; Heating; | |
| With sodium hydride In N,N-dimethyl-formamide 1.) 30 min., -10 to 0 deg C, 2.) 4h, room temperature; | |
| In 5,5-dimethyl-1,3-cyclohexadiene at 140℃; for 5h; Temperature; Large scale; |
-
-
60731-73-9
2,6-difluorobenzoyl isocyanate

-
-
50882-28-5
phenyl N-(4-chlorophenyl)carbamate

-
A
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

-
-
106-47-8
4-chloro-aniline

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 51.6 percent / aq. NaOH / dioxane / 15 h / 20 °C 2: 1,2-dichloro-ethane / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 69 percent / pyridine / tetrahydrofuran / 0.5 h / 20 °C 2: 1,2-dichloro-ethane / 3 h / 20 °C View Scheme |

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: benzene / 96 h / Ambient temperature 2: 70 percent / benzene / 11 h / Heating 3: 70 percent / Et3N / benzene / 2 h / Heating View Scheme |
-
-
148931-10-6
O-allyl N-(2,6-difluorobenzoyl)monothiocarbamate

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 70 percent / benzene / 11 h / Heating 2: 70 percent / Et3N / benzene / 2 h / Heating View Scheme |
-
-
18063-02-0
2,6-difluorobenzoylchloride

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) CH3CN, RT, 5 min, 2.) CH3CN, reflux, 5 h 2: 70 percent / Et3N / benzene / 2 h / Heating View Scheme |
-
-
280-57-9
1,4-diaza-bicyclo[2.2.2]octane

-
-
18063-03-1
2,6-difluorobenzamide

-
-
104-12-1
p-chlorphenylisocyanate

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In methanol; 1,2-dichloro-ethane |

-
-
104-12-1
p-chlorphenylisocyanate

-
-
79-34-5
1,1,2,2-tetrachloroethane

-
A
-
18063-03-1
2,6-difluorobenzamide

-
B
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| In n-heptane |

-
-
69385-30-4
2,6-difluorobenzylamine

-
-
104-12-1
p-chlorphenylisocyanate

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dichloromethane / 0.17 h / 0 - 20 °C 2: 9-(2-mesityl)-10-methylacridinium perchlorate / chloroform / 4 h / 35 °C / Irradiation View Scheme |
-
-
26028-43-3
1,3,5-triallylhexahydro-1,3,5-triazine

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-triallylhexahydro-1,3,5-triazine With phosphorus pentachloride In dichloromethane at 40℃; for 2.5h; Stage #2: 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea In dichloromethane at 1℃; for 0.25h; Stage #3: With triethylamine In dichloromethane at 1 - 20℃; for 5h; | 75% |

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea With sodium hydride In DMF (N,N-dimethyl-formamide) at 3℃; for 0.5h; Stage #2: PhCH2O2CN(CH2Cl)2 In tetrahydrofuran; DMF (N,N-dimethyl-formamide) at 1 - 20℃; for 6h; | 46.6% |
-
-
98025-72-0
bis-iodomethyl sulfide

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

-
-
596848-90-7
3-(4-chlorophenyl)-5-(2,6-difluorobenzoyl)tetrahydro-4H-1,3,5-thiadiazin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea With sodium hydride In DMF (N,N-dimethyl-formamide) at 3℃; for 0.666667h; Stage #2: bis-iodomethyl sulfide In DMF (N,N-dimethyl-formamide) at 3 - 20℃; for 18h; | 20% |
-
-
108-74-7
1,3,5-trimethyl-1,3,5-triazacyclohexane

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-trimethyl-1,3,5-triazacyclohexane With phosphorus pentachloride In dichloromethane at 40℃; for 2.5h; Stage #2: 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea In dichloromethane at 1℃; for 0.25h; Stage #3: With sodium hydroxide; triethylamine more than 3 stages; | 19% |
-
-
542-88-1
bis(2-chloromethyl)ether

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea With sodium hydride In DMF (N,N-dimethyl-formamide) at 3℃; for 0.666667h; Stage #2: bis(2-chloromethyl)ether In DMF (N,N-dimethyl-formamide) at 3 - 20℃; for 2h; | 19% |
-
-
10556-98-6
1,3,5-Triisopropyl-1,3,5-triazacyclohexane

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-Triisopropyl-1,3,5-triazacyclohexane With phosphorus pentachloride In dichloromethane at 40℃; for 4h; Stage #2: 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea In dichloromethane at 1℃; for 0.25h; Stage #3: With sodium hydroxide; triethylamine more than 3 stages; | 16% |
-
-
10556-98-6
1,3,5-Triisopropyl-1,3,5-triazacyclohexane

-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-Triisopropyl-1,3,5-triazacyclohexane With phosphorus pentachloride In dichloromethane at 40℃; for 3h; Stage #2: 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea In dichloromethane at 1℃; for 0.25h; Stage #3: With sodium hydroxide; triethylamine more than 3 stages; | 15% |
-
-
35367-38-5
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea

-
-
124-41-4
sodium methylate

-
A
-
13671-00-6
methyl 2,6-difluorobenzoate

-
B
-
140-38-5
N-(4-chlorophenyl)urea

| Conditions | Yield |
|---|---|
| In methanol at 25℃; Kinetics; Mechanism; Rate constant; |

Diflubenzuron Consensus Reports
Diflubenzuron Specification
The Diflubenzuron with CAS registry number of 35367-38-5 is also called 1-(4-chlorophenyl)-3-(2,6-ifluorobenzoyl)urea. Its EINECS registry number is 252-529-3. The IUPAC name is N-[(4-chlorophenyl)carbamoyl]-2,6-difluorobenzamide. In addition, the molecular formula is C14H9ClF2N2O2 and the molecular weight is 310.68. It belongs to the classes of Insecticide; Alphabetic; D; Growth regulatorsPesticides&Metabolites; Insecticides; Others; Pesticides; Alpha sort; DAlphabetic; Pesticides&Metabolites.
Physical properties about this chemical are: (1)ACD/LogP: 3.68; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.67; (4)ACD/LogD (pH 7.4): 3.53; (5)ACD/BCF (pH 5.5): 365.08; (6)ACD/BCF (pH 7.4): 262.47; (7)ACD/KOC (pH 5.5): 2372.15; (8)ACD/KOC (pH 7.4): 1705.46; (9)#H bond acceptors: 4; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 40.62 ?2; (13)Index of Refraction: 1.618; (14)Molar Refractivity: 73.96 cm3; (15)Molar Volume: 211.1 cm3; (16)Polarizability: 29.32 10-24cm3; (17)Surface Tension: 53 dyne/cm; (18)Density: 1.471 g/cm3.
Preparation of Diflubenzuron: it can be prepared by 4-chloro-aniline and N-(bis-methylsulfanyl-methylene)-2,6-difluoro-benzamide. This reaction will need reagent acetic acid. The reaction time is 6 hours by heating. The yield is about 69%.
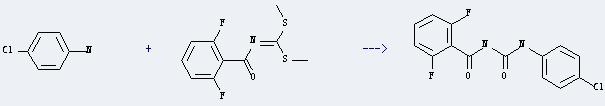
Uses of Diflubenzuron: It is an insect growth regulator which interferes with the formation of the insect cuticle. It is effective in the control of mosquitoes and flies. And it can be used to kill the armyworms of corn and wheat. In addition, it is used in forest management and on field crops to selectively control insect pests, particularly forest tent caterpillar moths, boll weevils, gypsy moths, and other types of moths.
When you are using this chemical, please be cautious about it as the following:
This chemical is very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. This material and/or its container must be disposed of as hazardous waste. You should avoid release to the environment. Moreover, you can refer to special instructions safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: Clc2ccc(NC(=O)NC(=O)c1c(F)cccc1F)cc2
(2)InChI: InChI=1/C14H9ClF2N2O2/c15-8-4-6-9(7-5-8)18-14(21)19-13(20)12-10(16)2-1-3-11(12)17/h1-7H,(H2,18,19,20,21)
(3)InChIKey: QQQYTWIFVNKMRW-UHFFFAOYAU
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 2150mg/kg (2150mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 968, 1986. | |
| mouse | LD50 | oral | 4640mg/kg (4640mg/kg) | Special Publication of the Entomological Society of America. Vol. 78-1, Pg. 20, 1978. | |
| mouse | LD50 | skin | > 6200mg/kg (6200mg/kg) | Nippon Noyaku Gakkaishi. Journal of the Pesticide Science Society of Japan. Vol. 17, Pg. S159, 1992. | |
| mouse | LD50 | subcutaneous | > 4gm/kg (4000mg/kg) | Nippon Noyaku Gakkaishi. Journal of the Pesticide Science Society of Japan. Vol. 17, Pg. S159, 1992. | |
| mouse | LD50 | unreported | 4600mg/kg (4600mg/kg) | Tsitologiya i Genetika. Cytology and Genetics. For English translation, see CYGEDX. Vol. 16(1), Pg. 45, 1982. | |
| rabbit | LD50 | skin | 2gm/kg (2000mg/kg) | Special Publication of the Entomological Society of America. Vol. 74-1, Pg. -, 1974. | |
| rat | LC | inhalation | > 35gm/m3/6H (35000mg/m3) | "Agrochemicals Handbook," with updates, Hartley, D., and H. Kidd, eds., Nottingham, Royal Soc of Chemistry, 1983-86Vol. A148, Pg. 1984, | |
| rat | LD50 | intraperitoneal | > 7500mg/kg (7500mg/kg) | Nippon Noyaku Gakkaishi. Journal of the Pesticide Science Society of Japan. Vol. 17, Pg. S159, 1992. | |
| rat | LD50 | oral | 4640mg/kg (4640mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 968, 1986. | |
| rat | LD50 | skin | > 10gm/kg (10000mg/kg) | Farm Chemicals Handbook. Vol. -, Pg. C106, 1991. | |
| rat | LD50 | subcutaneous | > 3400mg/kg (3400mg/kg) | Nippon Noyaku Gakkaishi. Journal of the Pesticide Science Society of Japan. Vol. 17, Pg. S159, 1992. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View