-
Name
Diisobutylaluminium hydride
- EINECS 214-729-9
- CAS No. 1191-15-7
- Article Data11
- CAS DataBase
- Density 0.848 g/cm3
- Solubility vigorous reaction with water, soluble in most organic solvents
- Melting Point -70 °C
- Formula C8H19Al
- Boiling Point 110 °C
- Molecular Weight 142.22
- Flash Point 4 °C
- Transport Information UN 3399 4.3/PG 1
- Appearance Clear solution
- Safety 26-43-45-62-46-36/37/39-29-16-61-27
- Risk Codes 14/15-17-35-40-67-65-63-48/20-11-19-62-51/53-23/24/25-20/21/22
-
Molecular Structure
-
Hazard Symbols
 T,
T, F,
F, C,
C, N
N
- Synonyms Aluminum,hydrodiisobutyl- (8CI);Diisobutylaluminum hydride (6CI);Bis(iso-butyl)aluminum hydride;Bis(isobutyl)aluminum hydride;Bis(isobutyl)hydroaluminum;DIBAH;DIBAL-H;Di-iso-butylaluminum hydride;Dibal;Diisobutylalane;Diisobutylaluminium hydride;Diisobutylhydroaluminum;Hydrodiisobutylaluminum;
- PSA 0.00000
- LogP 3.33250
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; diisobutylaluminium hydride In tetrahydrofuran; methanol | |
| With hydrogenchloride; diisobutylaluminium hydride In tetrahydrofuran; methanol | |
| With hydrogenchloride; diisobutylaluminium hydride In tetrahydrofuran; methanol |
-
-
100-99-2
triisobutylaluminum

-
A
-
5587-58-6
diisobutyl aluminium (1+); isobutylate

-
B
-
1191-15-7
diisobutylaluminium hydride

| Conditions | Yield |
|---|---|
| With water bubbling moistened helium stream (O2<0.001%) through Al-compd. (Drechsel vessel, room temp.); gas-liquid chromatographic anal., mass spectroscopy; |

| Conditions | Yield |
|---|---|
| In (2)H8-toluene toluene-d7, 1 equiv of Al-compound, 25°C; not sepd., detected by NMR spectra; |


| Conditions | Yield |
|---|---|
| 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 22°C, 2 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); α:β= >98:2; | A n/a B 2% C n/a |


-
-
1191-15-7
diisobutylaluminium hydride

| Conditions | Yield |
|---|---|
| 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 22°C, 2 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); α:β=>98:2; | A n/a B 2% C n/a |

-
-
67237-53-0
3-ethynylthiophene

-
-
1191-15-7
diisobutylaluminium hydride

| Conditions | Yield |
|---|---|
| 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 22°C, 2 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); α:β= >98:2; | A n/a B 2% C n/a |


| Conditions | Yield |
|---|---|
| 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 4°C, 12 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); α:β= 95:5; | A n/a B n/a C 2% |


| Conditions | Yield |
|---|---|
| 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 22°C, 2 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); α:β= >98:2; | A n/a B 2% C n/a |


| Conditions | Yield |
|---|---|
| 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 4°C, 12 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); α:β=98:2; | A n/a B 2% C n/a |


| Conditions | Yield |
|---|---|
| 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 4°C, 12 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); α:β=>98:2; | A n/a B 2% C n/a |


| Conditions | Yield |
|---|---|
| 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 22°C, 2 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); α:β= 97:3; | A n/a B n/a C 2% |


| Conditions | Yield |
|---|---|
| 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 22°C, 2 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); α:β= >98:2; | A n/a B 2% C n/a |


| Conditions | Yield |
|---|---|
| bis(diphenylphosphino)butane nickel(II) dichloride In tetrahydrofuran byproducts: C6H5CHCHC(CH2)C6H5; 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 22°C, 2 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); | A n/a B n/a C 2% |
| nickel(II) chloride hexahydrate In tetrahydrofuran byproducts: C6H5CHCHC(CH2)C6H5; 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 22°C, 2 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); | A n/a B n/a C 2% |
| nickel(II) acetylacetonate In tetrahydrofuran byproducts: C6H5CHCHC(CH2)C6H5; 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 22°C, 2 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); | A n/a B n/a C 2% |


-
-
1191-15-7
diisobutylaluminium hydride

| Conditions | Yield |
|---|---|
| 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 22°C, 2 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); α:β= >98:2; | A n/a B 2% C n/a |


| Conditions | Yield |
|---|---|
| 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran 3 mol% of Ni-catalyst, 1.3 equiv of Al-compound, THF, 4°C, 12 h, N2 atm.; detected by NMR spectra after quench with D2O (0°C, 30 min); α:β= >98:2; | A n/a B n/a C 2% |

-
-
204203-14-5
N-(2,6-diisopropylphenyl)-N-((1E)-1-(6-[(1E)-N-(2,6-diisopropylphenyl)ethanimidoyl]pyridin-2-yl)ethylidene)amine

-
-
1191-15-7
diisobutylaluminium hydride

-
A
-
879935-67-8
[Al(CH2CH(CH3)2)2(C5H4N(CCH3NC6H3(CH(CH3)2)2)2)]

| Conditions | Yield |
|---|---|
| In hexane under Ar; AlH(i-Bu)2 added to soln. of ligand in hexane, cooling, crystals of mono-Al complex isolated, soln. heated at 110°C for 2 h; cooling overnight yields crystals of di-Al complex; elem. anal.; | A n/a B 10.7% |

-
-
1191-15-7
diisobutylaluminium hydride

-
-
87884-41-1
2-cyano-6-styrylpyridine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; toluene | 17% |
-
-
111-66-0
oct-1-ene

-
-
1191-15-7
diisobutylaluminium hydride

-
-
4145-75-9
octylidenebis(diisobutylaluminium)

| Conditions | Yield |
|---|---|
| zirconocene dichloride In benzene Kinetics; (Ar); mixture of Zr complex, olefine and soln. of organoaluminum compd. diluted with benzene, stirred at 20 °C for 5 h; | 17% |
| With hydrogenchloride; meso-Me2C(2-Me-4-But-C5H2)2ZrCl2 In water; benzene at 0 - 20℃; Reagent/catalyst; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| zirconocene dichloride In benzene (Ar); mixture of Zr complex, olefine and soln. of organoaluminum compd. diluted with benzene, stirred at 20 °C for 5 h; | 17% |

| Conditions | Yield |
|---|---|
| Stage #1: diisobutylaluminium hydride With isopropyl alcohol In toluene at 25℃; Stage #2: C24H28O9 In toluene at 25 - 60℃; Sealed tube; | 17% |

| Conditions | Yield |
|---|---|
| zirconocene dichloride In benzene (Ar); mixture of Zr complex, olefine and soln. of organoaluminum compd. diluted with benzene, stirred at 20 °C for 5 h; | 18% |

| Conditions | Yield |
|---|---|
| zirconocene dichloride In benzene (Ar); mixture of Zr complex, olefine and soln. of organoaluminum compd. diluted with benzene, stirred at 20 °C for 5 h; | 18% |
-
-
5542-49-4
piperidin-1-yl benzoate

-
-
1191-15-7
diisobutylaluminium hydride

-
-
536-74-3
phenylacetylene

-
-
14990-66-0
1-(1-phenylvinyl)piperidine

| Conditions | Yield |
|---|---|
| Stage #1: diisobutylaluminium hydride; phenylacetylene With bis(triphenylphosphine)nickel(II) chloride In tetrahydrofuran at 22℃; for 3h; Inert atmosphere; Stage #2: piperidin-1-yl benzoate With copper(l) chloride In tetrahydrofuran at 22℃; for 1h; Inert atmosphere; | 18% |


| Conditions | Yield |
|---|---|
| zirconocene dichloride In benzene (Ar); mixture of Zr complex, olefine and soln. of organoaluminum compd. diluted with benzene, stirred at 20 °C for 5 h; | 19% |
| With hydrogenchloride; meso-Me2C(2-Me-4-But-C5H2)2ZrCl2 In water; benzene at 0 - 20℃; Reagent/catalyst; Inert atmosphere; |
-
-
3857-83-8
2-naphthyl triflate

-
-
1191-15-7
diisobutylaluminium hydride

-
A
-
612-78-2
2,2'-binaphthalene

-
B
-
26490-07-3
2-iso-butylnaphthalene

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride; trans-bis(triphenylphosphine)palladium dichloride In tetrahydrofuran Ambient temperature; | A 10% B 20% |
| trans-bis(triphenylphosphine)palladium dichloride In tetrahydrofuran Ambient temperature; | A 10% B 20% |


| Conditions | Yield |
|---|---|
| In hexane byproducts: KAl(i-Bu)3H, KAl(i-Bu)2H2, Al; mixed at -30°C, stirred (36 h, 20°C); filtered (Ar), residue washed (abs. ether), filtrate distilled off, hexane was added; elem. anal.; | 20% |

| Conditions | Yield |
|---|---|
| In hexane under N2 atm. to allyl methyl ether was added dropwise soln. i-Bu2AlH inn-hexane at room temp., react. mixt. was heated to 45°C, soln. i -Bu2AlH was added and refluxed for 4 h; solvent was removed in vacuo, residue was distilled fractionally; elem. anal.; | 20% |
-
-
31039-85-7
1-(2',4',6'-tri-tert-butylphenyl)acetylene

-
-
1191-15-7
diisobutylaluminium hydride

| Conditions | Yield |
|---|---|
| In pentane at 20℃; for 12h; Inert atmosphere; | 20% |

| Conditions | Yield |
|---|---|
| In diethyl ether; hexane at -78℃; for 2h; Schlenk technique; | 21% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; cyclohexane at 20℃; Inert atmosphere; Schlenk technique; Glovebox; | 23% |
Diisobutylaluminium hydride Consensus Reports
Reported in EPA TSCA Inventory.
Diisobutylaluminium hydride Standards and Recommendations
ACGIH TLV: TWA 2 mg(Al)/m3
Diisobutylaluminium hydride Specification
The Diisobutylaluminium hydride, with the CAS registry number 1191-15-7, is also known as Hydrobis(2-methylpropyl)aluminum. It belongs to the product categories of Organometallics; Al (Alminum) Compounds; Biochemistry; Classes of Metal Compounds; Reagents for Oligosaccharide Synthesis; Reduction; Synthetic Organic Chemistry; Typical Metal Compounds; Metal alkyl. Its EINECS number is 214-729-9. This chemical's molecular formula is C8H19Al and molecular weight is 142.22. What's more, its systematic name is hydrido[bis(2-methylpropyl)]aluminum. It should be sealed and stored at the temperature of 2 - 8 °C. Moreover, it should be protected from oxides, heat and fire. It is a prostaglandin reducing agent. It is mainly used as reducing agent and hydrogen aluminum agent in the fine chemicals. It is useful in organic synthesis for a variety of reductions, including converting esters and nitriles to aldehydes.
Preparation of Diisobutylaluminium hydride:
Diisobutylaluminium hydride can be prepared by by thermal decomposition in the reduced pressure and at the temperature of 120- 180 °C with triisobutyl aluminium as raw materials. The yield is up to 95%.
Uses of Diisobutylaluminium hydride:
It can be used to produce O2,O3;O5,O6-diisopropyliden-1,4-anhydro-D-mannitol at the temperature of 0 °C. It will need solvent toluene with the reaction time of 10 min. The yield is about 71%.
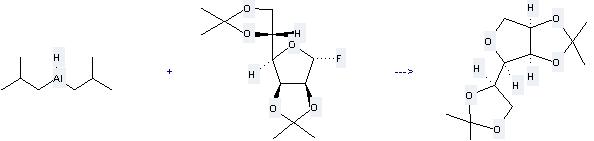
When you are using Diisobutylaluminium hydride, please be cautious about it as the following:
Diisobutylaluminium hydride is harmful by inhalation, in contact with skin and if swallowed. If swallowed, it will not induce vomiting that you need seek medical advice immediately and show this container or label. It is harmful as it may cause lung damage if swallowed and has a danger of serious damage to health by prolonged exposure through inhalation. This chemical is toxic by inhalation, in contact with skin and if swallowed. Moreover, it is toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. The substance will react violently with water, liberating extremely flammable gases. Its vapours may cause drowsiness and dizziness. This chemical is spontaneously flammable in air and may form explosive peroxides. It can cause severe burns. It has a limited evidence of a carcinogenic effect and has a possible risk of harm to the unborn child and a possible risk of impaired fertility. This chemical is highly flammable, so you should keep it away from sources of ignition - No smoking. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection. You must take off immediately all contaminated clothing. You should not empty it into drains. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible). You should avoid releasing it to the environment just refering to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: CC(C)C[AlH]CC(C)C
(2)Std. InChI:InChI=1S/2C4H9.Al.H/c2*1-4(2)3;;/h2*4H,1H2,2-3H3;
(3)Std. InChIKey: AZWXAPCAJCYGIA-UHFFFAOYSA-N
The toxicity data of Diisobutylaluminium hydride is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LCLo | inhalation | 70gm/m3/1H (70000mg/m3) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Medicina del Lavoro. Industrial Medicine. Vol. 57, Pg. 188, 1966. |
Related Products
- Diisobutylaluminium hydride
- 1191-16-8
- 119-12-0
- 1191-25-9
- 119126-15-7
- 119-13-1
- 119138-29-3
- 1191385-19-9
- 119139-23-0
- 119141-88-7
- 119141-89-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View