-
Name
Ergosterol
- EINECS 200-352-7
- CAS No. 57-87-4
- Article Data33
- CAS DataBase
- Density 1 g/cm3
- Solubility PRACTICALLY INSOLUBLE
- Melting Point 156-158 °C(lit.)
- Formula C28H44O
- Boiling Point 501.5 °C at 760 mmHg
- Molecular Weight 396.657
- Flash Point 216.3 °C
- Transport Information UN 2811 6.1/PG 2
- Appearance white to yellow crystalline powder
- Safety 28-36/37-45
- Risk Codes 28-48/20/22-40-38
-
Molecular Structure
-
Hazard Symbols
 T+
T+
- Synonyms Ergosterol (8CI);(22E,24R)-Ergosta-5,7,22-trien-3b-ol;(24R)-Ergosta-5,7,22-trien-3b-ol;24-Methylcholesta-5,7,22-trien-3b-ol;24R-Methylcholesta-5,7,22E-trien-3b-ol;24a-Methyl-22E-dehydrocholesterol;3b-Hydroxyergosta-5,7,22-triene;Ergosterin;Provitamin D2;Reishi Mushroom PE;
- PSA 20.23000
- LogP 7.33080
Synthetic route

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran for 16h; Heating; | 76% |
-
-
28421-56-9
3β-acetoxy-5α,8α-(4-phenyl-1,2,4-triazolidine-3,5-dione-1,2-diyl)ergosta-6,22-diene

-
A
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In hexane; toluene at 0℃; for 0.166667h; | A 70% B n/a |
-
-
10123-90-7
3β-hydroxy-3',5'-dioxo-4'-phenyl-5,8<1',2'>-1',2',4'-triazolidino-5α,8α-ergosta-6,22-diene

-
A
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In hexane; toluene at 0℃; for 0.166667h; | A 51% B n/a |
-
-
131321-88-5
(24R)-5,7-Ergostadiene-1α,3β,25-triol

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether for 0.05h; Irradiation; | 25% |
-
-
60-29-7
diethyl ether

-
-
21307-05-1
previtamin D2

-
A
-
57-87-4
Ergosterol

-
B
-
474-69-1
lumisterol

-
C
-
115-61-7
tachysterol

| Conditions | Yield |
|---|---|
| at 0℃; UV-Licht.Irradiation; |

-
-
17398-57-1
(22E,24R)-ergosta-4,7,22-triene-3-one

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With aluminum isopropoxide; isopropyl alcohol |
-
-
19044-12-3
lanosterol

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| biosynthesis by Botrytis cinerea in the presence of (dl)-cis-1-(4-chlorophenyl)-2-(1H-1,2,4-triazol-1-yl)cycloheptanol; |
-
-
87530-75-4
benzoyloxy-3β (urazolo-1',2')-5α,8α ergostadiene-6,22

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water for 2h; Heating; | 10 % Turnov. |
| With potassium hydroxide In ethanol for 2h; Heating; | 10 % Turnov. |
-
-
87530-76-5
benzoyloxy-3β (benzalamino-4' urazolo-1',2')-5α,8α ergostadiene-6,22

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water for 2h; Heating; | 53 % Turnov. |
| With potassium hydroxide In ethanol for 2h; Heating; | 53 % Turnov. |
-
-
87530-79-8
benzoyloxy-3β (p-nitrophenyl-4' urazolo-1',2')-5α,8α ergostadiene-6,22

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water for 2h; Heating; | 98 % Turnov. |
| With potassium hydroxide In ethanol for 2h; Heating; | 98 % Turnov. |
-
-
87530-77-6
benzoyloxy-3β (anisalamino-4' urazolo-1',2')-5α,8α ergostadiene-6,22

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water for 2h; Heating; | 29 % Turnov. |
| With potassium hydroxide In ethanol for 2h; Heating; | 29 % Turnov. |
| Multi-step reaction with 2 steps 1: 98 percent / NaNO2 (10percent) / tetrahydrofuran; acetic acid 2: 10 percent Turnov. / KOH (0.8N) / ethanol / 2 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: 98 percent / 2,4-dinitro phenylhydrazine, p-toluenesulfonic acid monohydrate / H2O; tetrahydrofuran / 3 h / Heating 2: 18 percent Turnov. / KOH (0.8N) / ethanol / 2 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: 98 percent / 2,4-dinitro phenylhydrazine, p-toluenesulfonic acid monohydrate / H2O; tetrahydrofuran / 3 h / Heating 2: 95 percent / PhSeOOH / tetrahydrofuran / 0.33 h / Heating 3: 10 percent Turnov. / KOH (0.8N) / ethanol / 2 h / Heating View Scheme |
-
-
87530-78-7
benzoyloxy-3β (p-nitrobenzalamino-4' urazolo-1',2')-5α,8α ergostadiene-6,22

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water for 2h; Heating; | 67 % Turnov. |
| With potassium hydroxide In ethanol for 2h; Heating; | 62 % Turnov. |
-
-
104729-32-0
benzoyloxy-3β (amino-4' urazolo-1',2')-5α,8α ergostadiene-6,22

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 2h; Heating; | 18 % Turnov. |
| Multi-step reaction with 2 steps 1: 95 percent / PhSeOOH / tetrahydrofuran / 0.33 h / Heating 2: 10 percent Turnov. / KOH (0.8N) / ethanol / 2 h / Heating View Scheme |
-
-
28487-73-2, 76035-90-0
(22E)-5α,8α-(4'-phenyl)urazoloergosta-6,22-dien-3β-yl benzoate

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water for 2h; Heating; | 37 % Turnov. |
| With potassium hydroxide In ethanol for 2h; Heating; | 37 % Turnov. |
-
-
24352-51-0
(22E)-3α,5-cyclo-5α-ergosta-6,8(14),22-triene

-
-
76-03-9
trichloroacetic acid

-
A
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| at 80℃; Kochen des Reaktionsprodukts mit aethanol. NaOH; |

-
-
67-66-3
chloroform

-
-
24352-51-0
(22E)-3α,5-cyclo-5α-ergosta-6,8(14),22-triene

-
-
76-03-9
trichloroacetic acid

-
A
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| at 20℃; Kochen des Reaktionsprodukts mit aethanol. NaOH; |

-
-
71-23-8
propan-1-ol

-
-
516-85-8
9(11)-dehydroergosterol

-
A
-
57-87-4
Ergosterol

-
B
-
516-84-7
ergosta-7,9(11),22-triene-3β-ol

-
C
-
2465-11-4
(22E)-ergosta-7,22-dien-3β-ol

-
-
64-17-5
ethanol

-
-
516-85-8
9(11)-dehydroergosterol

-
A
-
57-87-4
Ergosterol

-
B
-
516-84-7
ergosta-7,9(11),22-triene-3β-ol

-
C
-
2465-11-4
(22E)-ergosta-7,22-dien-3β-ol

-
-
17398-57-1
(22E,24R)-ergosta-4,7,22-triene-3-one

-
-
555-31-7
aluminum isopropoxide

-
-
67-63-0
isopropyl alcohol

-
A
-
57-87-4
Ergosterol

-
B
-
6538-05-2
24βF-methyl-cholestatrien-(4.7.22t)-ol-(3α)

-
C
-
97583-19-2
ergosta-4,7,22-trien-3-ol

-
-
125315-55-1
22-hydroxy-5α,8α-(4-phenyl-3,5-dioxo-1,2,4-triazolidine-1,2-diyl)-23,24-dinorchol-6-ene-1α,3β-diol 1,3-diacetate

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: pyridine / 4 h / Ambient temperature 2: 1) NaI / 1) DMF, 30 min, 80 deg C, 2) 30 min, 80 deg C 3: 79 percent / KOH / methanol / 0.5 h / Heating 4: 90 percent / PPTS / CH2Cl2 / 24 h / Ambient temperature 5: 1) BuLi, HMPA / 1) THF, -78 deg C to -20 deg C, 20 min, 2) THF, -20 deg C, 1.5h 6: 53 percent / sodium amalgam (5percent), Na2HPO4 / methanol / 16 h / Ambient temperature 7: 83 percent / p-TsOH*H2O, 95percent EtOH / 4 h / 80 °C 8: 86 percent / LiAlH4 / tetrahydrofuran / Heating 9: 25 percent / diethyl ether; tetrahydrofuran / 0.05 h / Irradiation View Scheme |
-
-
125315-53-9
22-Oxo-5α,8α-(4-phenyl-3,5-dioxo-1,2,4-triazolidine-1,2-diyl)-23,24-dinor-6-cholene-1α,3β-diyl diacetate

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 950 g / NaBH4 / methanol / 0.17 h / Ambient temperature 2: pyridine / 4 h / Ambient temperature 3: 1) NaI / 1) DMF, 30 min, 80 deg C, 2) 30 min, 80 deg C 4: 79 percent / KOH / methanol / 0.5 h / Heating 5: 90 percent / PPTS / CH2Cl2 / 24 h / Ambient temperature 6: 1) BuLi, HMPA / 1) THF, -78 deg C to -20 deg C, 20 min, 2) THF, -20 deg C, 1.5h 7: 53 percent / sodium amalgam (5percent), Na2HPO4 / methanol / 16 h / Ambient temperature 8: 83 percent / p-TsOH*H2O, 95percent EtOH / 4 h / 80 °C 9: 86 percent / LiAlH4 / tetrahydrofuran / Heating 10: 25 percent / diethyl ether; tetrahydrofuran / 0.05 h / Irradiation View Scheme |
-
-
131249-29-1
22-Phenylsulfonyl-5α,8α-(4-phenyl-3,5-dioxo-1,2,4-triazolidine-1,2-diyl)-23,24-dinor-6-cholene-1α,3β-diol

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 90 percent / PPTS / CH2Cl2 / 24 h / Ambient temperature 2: 1) BuLi, HMPA / 1) THF, -78 deg C to -20 deg C, 20 min, 2) THF, -20 deg C, 1.5h 3: 53 percent / sodium amalgam (5percent), Na2HPO4 / methanol / 16 h / Ambient temperature 4: 83 percent / p-TsOH*H2O, 95percent EtOH / 4 h / 80 °C 5: 86 percent / LiAlH4 / tetrahydrofuran / Heating 6: 25 percent / diethyl ether; tetrahydrofuran / 0.05 h / Irradiation View Scheme |
-
-
131249-28-0
22-Phenylsulfonyl-5α,8α-(4-phenyl-3,5-dioxo-1,2,4-triazolidine-1,2-diyl)-23,24-dinor-6-cholene-1α,3β-diyl Diacetate

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 79 percent / KOH / methanol / 0.5 h / Heating 2: 90 percent / PPTS / CH2Cl2 / 24 h / Ambient temperature 3: 1) BuLi, HMPA / 1) THF, -78 deg C to -20 deg C, 20 min, 2) THF, -20 deg C, 1.5h 4: 53 percent / sodium amalgam (5percent), Na2HPO4 / methanol / 16 h / Ambient temperature 5: 83 percent / p-TsOH*H2O, 95percent EtOH / 4 h / 80 °C 6: 86 percent / LiAlH4 / tetrahydrofuran / Heating 7: 25 percent / diethyl ether; tetrahydrofuran / 0.05 h / Irradiation View Scheme |
-
-
131249-26-8
5α,8α-(4-Phenyl-3,5-dioxo-1,2,4-triazolidine-1,2-diyl)-23,24-dinor-6-cholene-1α,3β,22-triyl 1α,3β-Diacetate 22-p-Toluenesulfonate

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 1) NaI / 1) DMF, 30 min, 80 deg C, 2) 30 min, 80 deg C 2: 79 percent / KOH / methanol / 0.5 h / Heating 3: 90 percent / PPTS / CH2Cl2 / 24 h / Ambient temperature 4: 1) BuLi, HMPA / 1) THF, -78 deg C to -20 deg C, 20 min, 2) THF, -20 deg C, 1.5h 5: 53 percent / sodium amalgam (5percent), Na2HPO4 / methanol / 16 h / Ambient temperature 6: 83 percent / p-TsOH*H2O, 95percent EtOH / 4 h / 80 °C 7: 86 percent / LiAlH4 / tetrahydrofuran / Heating 8: 25 percent / diethyl ether; tetrahydrofuran / 0.05 h / Irradiation View Scheme |
-
-
131249-30-4
22-Phenylsulfonyl-5α,8α-(4-phenyl-3,5-dioxo-1,2,4-triazolidine-1,2-diyl)-1α,3β-bis(tetrahydropyranyloxy)-23,24-dinor-6-cholene

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 1) BuLi, HMPA / 1) THF, -78 deg C to -20 deg C, 20 min, 2) THF, -20 deg C, 1.5h 2: 53 percent / sodium amalgam (5percent), Na2HPO4 / methanol / 16 h / Ambient temperature 3: 83 percent / p-TsOH*H2O, 95percent EtOH / 4 h / 80 °C 4: 86 percent / LiAlH4 / tetrahydrofuran / Heating 5: 25 percent / diethyl ether; tetrahydrofuran / 0.05 h / Irradiation View Scheme |
-
-
131249-35-9, 131321-86-3
(24R)-5α,8α-(4-Phenyl-3,5-dioxo-1,2,4-triazolidine-1,2-diyl)-1α,3β,25-tris(tetrahydropyranyloxy)-6-ergostene

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 83 percent / p-TsOH*H2O, 95percent EtOH / 4 h / 80 °C 2: 86 percent / LiAlH4 / tetrahydrofuran / Heating 3: 25 percent / diethyl ether; tetrahydrofuran / 0.05 h / Irradiation View Scheme |
-
-
131249-33-7
(24R)-22-Phenylsulfonyl-5α,8α-(4-phenyl-3,5-dioxo-1,2,4-triazolidine-1,2-diyl)-1α,3β,25-tris(tetrahydropyranyloxy)-6-ergostene

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 53 percent / sodium amalgam (5percent), Na2HPO4 / methanol / 16 h / Ambient temperature 2: 83 percent / p-TsOH*H2O, 95percent EtOH / 4 h / 80 °C 3: 86 percent / LiAlH4 / tetrahydrofuran / Heating 4: 25 percent / diethyl ether; tetrahydrofuran / 0.05 h / Irradiation View Scheme |
-
-
131249-25-7, 131321-85-2
(3S)-4-iodo-2,3-dimethyl-2-butanol tetrahydropyranyl ether

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 1) BuLi, HMPA / 1) THF, -78 deg C to -20 deg C, 20 min, 2) THF, -20 deg C, 1.5h 2: 53 percent / sodium amalgam (5percent), Na2HPO4 / methanol / 16 h / Ambient temperature 3: 83 percent / p-TsOH*H2O, 95percent EtOH / 4 h / 80 °C 4: 86 percent / LiAlH4 / tetrahydrofuran / Heating 5: 25 percent / diethyl ether; tetrahydrofuran / 0.05 h / Irradiation View Scheme |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 3h; Inert atmosphere; | 100% |
| With pyridine at 20℃; for 24h; | 96% |
| With pyridine; dmap In dichloromethane at 20℃; | 92% |
-
-
15988-11-1
4-Phenylurazole

-
-
57-87-4
Ergosterol

-
-
10123-90-7
3β-hydroxy-3',5'-dioxo-4'-phenyl-5,8<1',2'>-1',2',4'-triazolidino-5α,8α-ergosta-6,22-diene

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 15h; | 100% |
| With (PhSeO)2O In tetrahydrofuran for 2h; Ambient temperature; | 96% |

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide | 100% |
| With phosphorus pentoxide at 20℃; for 1h; | 89% |

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In methanol; dichloromethane at 20℃; for 20h; | 99% |
| With diethyl ether; palladium at 20℃; Hydrogenation; | |
| With diethyl ether; palladium Hydrogenation; |

| Conditions | Yield |
|---|---|
| With tert-Amyl alcohol; ammonia; lithium In tetrahydrofuran at -78℃; for 4h; | 99% |
| With diisobutylaluminium hydride In toluene at 110℃; for 6h; in sealed flask; | 94% |
| With diethyl ether; palladium Hydrogenation; |

| Conditions | Yield |
|---|---|
| With iron(III) chloride hexahydrate In 1,3,5-trimethyl-benzene for 24h; Reflux; | 99% |
| With enzyme-substance from pig's pancreas | |
| at 190℃; |

| Conditions | Yield |
|---|---|
| With iron(III) chloride hexahydrate In 1,3,5-trimethyl-benzene for 24h; Reflux; | 99% |
| With hydrogenchloride; sodium dodecyl-sulfate Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With iron(III) chloride hexahydrate In 1,3,5-trimethyl-benzene for 12h; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride hexahydrate In 1,3,5-trimethyl-benzene for 12h; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride hexahydrate In 1,3,5-trimethyl-benzene for 12h; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride hexahydrate In 1,3,5-trimethyl-benzene for 24h; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With pyridine; copper(l) chloride In tetrahydrofuran at 20℃; for 2h; | 99% |

| Conditions | Yield |
|---|---|
| With pyridine at 10℃; for 1h; Inert atmosphere; | 98% |
| With pyridine at 10℃; for 1h; | 94% |
| With pyridine at 10℃; for 1h; | 94% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane | 98% |
| With pyridine In dichloromethane at 0 - 20℃; for 18h; | 94% |
-
-
57-87-4
Ergosterol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
95307-26-9
3β-tert-Butyldimethylsilyloxy-ergosta-5,7,22-triene

| Conditions | Yield |
|---|---|
| With pyridine; 1H-imidazole In toluene at 110℃; for 3h; Inert atmosphere; | 98% |
| With pyridine; 1H-imidazole at 25℃; for 2h; Etherification; | 97% |
| With 1H-imidazole In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 24h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 10℃; for 18h; | 98% |
-
-
57-87-4
Ergosterol

-
-
23637-31-2
5α,6α-epoxy-24(R)-methylcholesta-7,22-dien-3β-ol

| Conditions | Yield |
|---|---|
| With potassium carbonate; 3-chloro-benzenecarboperoxoic acid In dichloromethane; water at 25℃; for 0.75h; diastereoselective reaction; | 98% |
| With potassium carbonate; 3-chloro-benzenecarboperoxoic acid In dichloromethane; water at 25℃; for 0.75h; Sonication; | 98% |
-
-
57-87-4
Ergosterol

-
-
13274-45-8
4-(p-nitrophenyl)urazole

-
-
87530-80-1
(p-nitrophenyl-4' urazolo-1',2')-5α,8α ergostadiene-6,22 ol-3β

| Conditions | Yield |
|---|---|
| With (PhSeO)2O In tetrahydrofuran for 2h; Ambient temperature; | 97% |
-
-
57-87-4
Ergosterol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
51373-27-4
(22E,24R)-5α-ergosta-5,7,22-triene-3-p-toluenesulfonate

| Conditions | Yield |
|---|---|
| With sodium carbonate In toluene at 10℃; for 2h; Reagent/catalyst; Temperature; | 96.3% |
-
-
57-87-4
Ergosterol

-
-
17608-76-3
(22E,24R)-ergosta-7,22-dien-3β-ol

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In toluene at 110℃; for 60h; | 96% |
| With lithium; ethylamine |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 24h; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 24h; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With pyridine for 6h; Ambient temperature; | 95% |
| With pyridine |

| Conditions | Yield |
|---|---|
| With calcium hydride; N-benzyl-N,N,N-triethylammonium chloride In toluene | 95% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.5h; nitroso-Diels-Alder reaction; stereoselective reaction; | 95% |
-
-
57-87-4
Ergosterol

-
-
151767-11-2
2,3,4,5-tetra-O-benzoyl-D-glucopyranosyl trichloroacetimidate

-
-
1354766-16-7
ergosterol-3-O-(2,3,4,6-tetra-O-benzoyl-β-D-glucopyranoside)

| Conditions | Yield |
|---|---|
| Stage #1: Ergosterol; 2,3,4,5-tetra-O-benzoyl-D-glucopyranosyl trichloroacetimidate In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Molecular sieve; Stage #2: With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0 - 20℃; for 1.5h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 24h; Inert atmosphere; | 95% |
-
-
2596-47-6, 147677-05-2, 34749-55-8
(E)-3-(4-acetoxy-3-methoxyphenyl)acrylic acid

-
-
57-87-4
Ergosterol

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 100℃; under 13501.4 Torr; for 0.25h; Microwave irradiation; | 94% |

| Conditions | Yield |
|---|---|
| With oxygen; phloxine B In methanol at 5℃; for 3h; Irradiation; Inert atmosphere; | 93% |
| With oxygen In methanol Diels-Alder Cycloaddition; UV-irradiation; | 93% |
| With oxygen; 5,10,15,20-tetraphenylporphyrin In dichloromethane at 0℃; for 4h; Irradiation; | 70% |
-
-
57-87-4
Ergosterol

-
-
203435-09-0
2,3,4,6-tetra-O-benzoyl-α,b-D-galactopyranosyl trichloroacetimidate

-
-
1390620-14-0
ergosterol-3-O-(2,3,4,6-tetra-O-benzoyl-β-D-galactopyranoside)

| Conditions | Yield |
|---|---|
| Stage #1: Ergosterol; 2,3,4,6-tetra-O-benzoyl-α,b-D-galactopyranosyl trichloroacetimidate In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Molecular sieve; Stage #2: With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0 - 20℃; for 1.5h; Inert atmosphere; | 93% |
Ergosterol Standards and Recommendations
OPTICAL ROTATION: -122° ~ -132° (C=1.2 in CHCl3)
Ergosterol Specification
The CAS registry number of Ergosterol is 57-87-4. Its EINECS registry number is 200-352-7. The IUPAC name is (3S,9S,10R,13R,14R,17R)-17-[(E,2R,5R)-5,6-dimethylhept-3-en-2-yl]-10,13-dimethyl-2,3,4,9,11,12,14,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3-ol. In addition, the molecular formula is C28H44O and the molecular weight is 396.65. What's more, it is a biological precursor (a provitamin) to vitamin D2 and belongs to the classes of Pharmaceutical Intermediates; Steroids; Biochemistry; Hydroxysteroids; Vitamins; Vitamins and derivatives.
Physical properties about this chemical are: (1)ACD/LogP: 9.30; (2)# of Rule of 5 Violations: 1; (3)#H bond acceptors: 1; (4)#H bond donors: 1; (5)#Freely Rotating Bonds: 5; (6)Polar Surface Area: 9.23 Å2; (7)Index of Refraction: 1.542; (8)Molar Refractivity: 124.19 cm3; (9)Molar Volume: 394 cm3; (10)Polarizability: 49.23 ×10-24cm3; (11)Surface Tension: 38.9 dyne/cm; (12)Density: 1 g/cm3; (13)Flash Point: 216.3 °C; (14)Enthalpy of Vaporization: 88.71 kJ/mol; (15)Boiling Point: 501.5 °C at 760 mmHg; (16)Vapour Pressure: 3.7E-12 mmHg at 25°C.
Preparation of Ergosterol: Ergosterol is present in cell membranes of fungi yet absent in those of animals. And it is also present in the cell membranes of some protists, such as trypanosomes. In addition, it can be extracted from yeast. And it also can be extracted from tobacco.
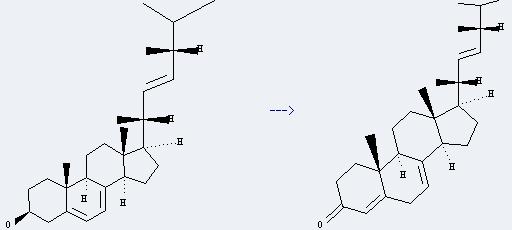
Uses of Ergosterol: it can be used in biochemical research and used as an indicator of fungal biomass in soil. And it can be used to get ergosterone. This reaction will need reagent aluminum triisopropoxide and solvents toluene and cyclohexanone. The reaction time is 20 minutes by heating. The yield is about 75%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to the skin and very toxic if swallowed. There is limited evidence of a carcinogenic effect . And it has danger of serious damage to health by prolonged exposure through inhalation and if swallowed. When you are using it, wear suitable protective clothing and gloves. After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer). In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: O[C@@H]4C/C3=C/C=C1\[C@H](CC[C@]2([C@H]1CC[C@@H]2[C@@H](/C=C/[C@H](C)C(C)C)C)C)[C@@]3(C)CC4
(2)InChI: InChI=1/C28H44O/c1-18(2)19(3)7-8-20(4)24-11-12-25-23-10-9-21-17-22(29)13-15-27(21,5)26(23)14-16-28(24,25)6/h7-10,18-20,22,24-26,29H,11-17H2,1-6H3/b8-7+/t19-,20+,22-,24+,25-,26-,27-,28+/m0/s1
(3)InChIKey: DNVPQKQSNYMLRS-APGDWVJJBI
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View