-
Name
Ethyl 4-chloro-2-methylthio-5-pyrimidinecarboxylate
- EINECS 227-619-0
- CAS No. 5909-24-0
- Article Data16
- CAS DataBase
- Density 1.37 g/cm3
- Solubility
- Melting Point 60-63 °C(lit.)
- Formula C8H9ClN2O2S
- Boiling Point 335.7 °C at 760 mmHg
- Molecular Weight 232.691
- Flash Point 156.83 °C
- Transport Information
- Appearance off white to light yellow solid
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Methylthio-4-chloro-5-ethoxycarbonylpyrimidine;4-Chloro-2-methanethiopyrimidine-5-carboxylic acid ethyl ester;4-Chloro-2-methylsulfanylpyrimidine-5-carboxylic acid ethyl ester;4-Chloro-5-carbethoxy-2-methylthiopyrimidine;4-Chloro-5-ethoxycarbonyl-2-methylthiopyrimidine;
- PSA 77.38000
- LogP 2.02860
Synthetic route
-
-
53554-29-3
ethyl 4-hydroxy-2-(methylthio)pyrimidine-5-carboxylate

-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With thionyl chloride at 60℃; for 3h; | 92% |
| With N,N-diethylaniline; trichlorophosphate for 5h; Heating / reflux; | 77% |
| With trichlorophosphate In acetonitrile for 6h; Reagent/catalyst; Reflux; | 77% |

-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: sodium 5-(ethoxycarbonyl)-2-(methylthio)-4-oxo-4H-pyrimidin-3-ide With thionyl chloride In N,N-dimethyl-formamide; toluene at 20 - 30℃; for 0.5h; Large scale; Stage #2: With trichlorophosphate In N,N-dimethyl-formamide; toluene at 55 - 65℃; for 1.5h; Large scale; | 84.5% |
| With trichlorophosphate for 3h; Reflux; | |
| With trichlorophosphate at 0℃; for 5h; Reflux; |
-
-
53554-29-3
ethyl 3,4-dihydro-2-methylthio-4-oxopyrimidine-5-carboxylate

-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With thionyl chloride | |
| With trichlorophosphate | |
| With trichlorophosphate at 100℃; for 3h; |
-
-
87-13-8
diethyl 2-ethoxymethylenemalonate

-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: EtONa / ethanol 2: POCl3 View Scheme | |
| Multi-step reaction with 2 steps 1: aqueous KOH 2: POCl3 View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium hydroxide / water / 0.08 h / 20 °C 1.2: 24 h / 20 °C 2.1: trichlorophosphate / 3 h / Reflux View Scheme |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
74-89-5
methylamine

-
-
76360-82-2
4-methylamino-2-methylsulfanyl-pyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In ethanol; dichloromethane at 0℃; for 0.5h; | 100% |
| With triethylamine In tetrahydrofuran at 50℃; | 100% |
| at 0℃; for 0.5h; | 100% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
117147-06-5
ethyl 4-hydrazinyl-2-(methylsulfanyl)pyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol at 0℃; for 1h; | 100% |
| With hydrazine hydrate In ethanol at 0℃; for 1h; | 91.8% |
| With hydrazine hydrate In ethanol at 0 - 20℃; for 1h; | 85.71% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
1003-03-8
Cyclopentamine

-
-
211245-62-4
4-cyclopentylamino-2-(methylsulfanyl)pyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| With triethylamine In dichloromethane for 0.75h; Heating / reflux; | 97% |
| With triethylamine In tetrahydrofuran at 20℃; for 1h; | 96.1% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
115514-77-7
(3-chloro-4-methoxyphenyl)methanamine

-
-
330785-81-4
ethyl 4-[(3-chloro-4-methoxybenzyl)amino]-2-(methylsulfanyl)pyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; | 100% |
| With triethylamine In acetone at 20℃; for 3h; | 87% |
| With triethylamine In dichloromethane at 20℃; for 10h; | 76% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
60295-21-8
N'-cyclohexyl-hydrazine carboxylic acid tert-butyl ester

-
-
900502-62-7
C19H30N4O4S

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; | 100% |
| With triethylamine In tetrahydrofuran at 20℃; | 100% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
1410975-83-5
N-(((2S,4R)-4-hydroxypyrrolidin-2-yl)methyl)-2-nitrobenzenesulfonamide

-
-
1410975-85-7
ethyl 4-((2S,4R)-4-hydroxy-2-((2-nitrophenylsulfonamido)methyl)pyrrolidin-1-yl)-2-(methylthio)pyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 90℃; for 1.5h; | 100% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
75-04-7
ethylamine

-
-
185040-33-9
4-ethylamino-2-methylsulfanyl-pyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 0 - 20℃; for 8h; | 99.1% |
| With triethylamine In tetrahydrofuran; water at 20℃; for 4h; | 93% |
| With triethylamine In tetrahydrofuran at 20℃; | 92% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
776-53-4
ethyl 4-amino-2-methylmercaptopyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; triethylamine In tetrahydrofuran; water at 30℃; for 2h; | 99% |
| With ammonium hydroxide; triethylamine In tetrahydrofuran for 2h; | 99% |
| With ammonium hydroxide; triethylamine In tetrahydrofuran at 30℃; for 2h; | 99% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
75-31-0
isopropylamine

-
-
25693-79-2
4-isopropylamino-2-(methylsulfanyl)pyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 0 - 20℃; for 8h; | 99% |
| In 1-methyl-pyrrolidin-2-one at 60℃; | 98% |
| With triethylamine In tetrahydrofuran at 20℃; | 95% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
2213-08-3
ethyl 3-methylaminopropionate

-
-
625106-58-3
ethyl 4-((3-ethoxy-3-oxopropyl)(methyl)amino)-2-(methylthio)pyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile for 16h; Reflux; | 99% |
| In tetrahydrofuran at 50℃; for 1h; Inert atmosphere; | 43% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
5452-35-7
cycloheptanamine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; for 18h; | 99% |
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 4h; | 90% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
73781-88-1
ethyl 2-(methylthio)pyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen; sodium carbonate In ethanol under 3102.97 Torr; for 48h; | 98.2% |
| With hydrogen; sodium hydrogencarbonate; palladium 10% on activated carbon In ethanol for 48h; | 70% |
| With acetic acid; zinc In tetrahydrofuran for 6h; Heating / reflux; | 63% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
617-79-8
2-ethyl-N-butylamine

-
-
686266-25-1
methyl 4-[(2-ethylbutyl)amino]-2-(methylthio)pyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine at 20℃; for 18h; | 98% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
593-51-1
methylamine hydrochloride

-
-
76360-82-2
4-methylamino-2-methylsulfanyl-pyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 15h; Inert atmosphere; | 98% |
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 15h; Inert atmosphere; | 94% |
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 100℃; for 3h; |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
1410975-65-3
N-(((2S,4R)-4-fluoropyrrolidine-2-yl)methyl)-2-nitrobenzenesulfonamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 90℃; for 2.5h; | 97% |
-
-
123652-95-9
1-(3-fluoro-4-methoxyphenyl)methanamine

-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 0.5h; | 97% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
100-46-9
benzylamine

-
-
100973-67-9
ethyl 4-(benzylamino)-2-(methylthio)pyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 5h; | 96% |
| With N-ethyl-N,N-diisopropylamine In acetonitrile | |
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 20℃; | |
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 0 - 26℃; |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
68621-88-5
(3-aminophenyl)carbamic acid tert-butyl ester

-
-
1363161-14-1
4-(3-tert-butoxycarbonylaminoanilino)-2-methylmercaptopyrimidine-5-carbonic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; Inert atmosphere; | 96% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; Inert atmosphere; | 96% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; | 95% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In ethanol at 60℃; for 2.5h; | 96% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 4 - 65℃; for 17h; | 95.5% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
955370-01-1
1-ethylhydrazinecarboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 60℃; for 16h; | 95.03% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 60℃; for 16h; | 95% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
765-30-0
Cyclopropylamine

-
-
651734-65-5
4-cyclopropylamino-2-(methylsulfanyl)pyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; | 95% |
| With triethylamine In acetonitrile at 0 - 20℃; for 4h; | 92% |
| With triethylamine In tetrahydrofuran at 20℃; | 90% |
| With triethylamine In tetrahydrofuran at 20℃; | 90% |
| With triethylamine In dichloromethane at 15 - 42℃; for 2h; Inert atmosphere; |

-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
185040-33-9
4-ethylamino-2-methylsulfanyl-pyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran | 95% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
1187160-18-4
1-amino-7-methoxyindane hydrochloride

-
-
1232030-41-9
ethyl 4-(7-methoxy-2,3-dihydro-1H-inden-1ylamino)-2-(methylthio)pyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; | 95% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
22287-35-0
1-aminobicyclo<1.1.1>pentane hydrochloride

-
-
1403865-19-9
ethyl 4-(bicyclo[1.1.1]pentan-1-ylamino)-2-(methylthio)pyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 48h; Inert atmosphere; | 95% |
| With triethylamine In tetrahydrofuran at 20℃; for 72h; | 80% |
| With N-ethyl-N,N-diisopropylamine In ethanol at 20℃; |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
109-73-9
N-butylamine

-
-
1027417-40-8
4-butylamino-2-methylsulfanyl-pyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; | 95% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
110-58-7
1-pentanamine

-
-
1537216-06-0
2-methylsulfanyl-4-pentylamino-pyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; | 95% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

-
-
6373-46-2
p-benzyloxyaniline

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dimethylsulfoxide-d6 at 20 - 80℃; for 2h; | 95% |
-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane for 0.5h; Heating; | 94% |
-
-
868733-71-5
4-(4-amino-2-fluorophenoxy)pyridin-2-carboxamide

-
-
5909-24-0
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; 1-methyl-pyrrolidin-2-one | 93% |
Ethyl 4-chloro-2-methylthio-5-pyrimidinecarboxylate Specification
The Ethyl 4-chloro-2-methylthio-5-pyrimidinecarboxylate, with the CAS registry number 5909-24-0 and EINECS registry number 227-619-0, has the systematic name of ethyl 4-chloro-2-(methylsulfanyl)pyrimidine-5-carboxylate. And the molecular formula of this chemical is C8H9ClN2O2S. It is a kind of off white to light yellow solid, and should keep cold. In addition, it is a pyrimidine derivative, and used as inhibitor of Streptococcus faecium, Lactobacillus casei, and Pediococcus cerevisiae.
The physical properties of Ethyl 4-chloro-2-methylthio-5-pyrimidinecarboxylate are as following: (1)ACD/LogP: 1.95; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.945; (4)ACD/LogD (pH 7.4): 1.945; (5)ACD/BCF (pH 5.5): 17.719; (6)ACD/BCF (pH 7.4): 17.719; (7)ACD/KOC (pH 5.5): 272.428; (8)ACD/KOC (pH 7.4): 272.428; (9)#H bond acceptors: 4; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 77.38 Å2; (13)Index of Refraction: 1.568; (14)Molar Refractivity: 55.559 cm3; (15)Molar Volume: 169.735 cm3; (16)Polarizability: 22.025×10-24cm3; (17)Surface Tension: 57.906 dyne/cm; (18)Density: 1.371 g/cm3; (19)Flash Point: 156.83 °C; (20)Enthalpy of Vaporization: 57.879 kJ/mol; (21)Boiling Point: 335.707 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
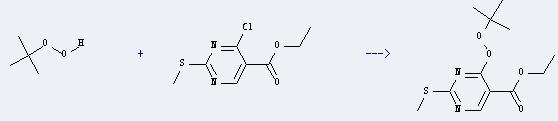
Uses of Ethyl 4-chloro-2-methylthio-5-pyrimidinecarboxylate: It can react with tert-butyl hydroperoxide to produce 4-tert-butylperoxy-2-methylsulfanyl-pyrimidine-5-carboxylic acid ethyl ester. This reaction will need reagent Bariumoxid, and the solvent petroleum ether. The reaction time is 2 hours with temperature of 0°C, and the yield is about 62%.
You should be cautious while dealing with this chemical. It irritates eyes, respiratory system and skin. Therefore, you had better take the following instructions: Wear suitable protective clothing, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: CCOC(=O)c1cnc(nc1Cl)SC
(2)InChI: InChI=1/C8H9ClN2O2S/c1-3-13-7(12)5-4-10-8(14-2)11-6(5)9/h4H,3H2,1-2H3
(3)InChIKey: SNNHLSHDDGJVDM-UHFFFAOYAH
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 59092-91-0
- 590-93-2
- 590-97-6
- 59101-27-8
- 59101-28-9
- 591-01-5
- 5910-25-8
- 5910-28-1
- 59104-19-7
- 59104-21-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View