-
Name
ETHYL5-ACETOXY-6-BROMO-2-(BROMOMETHYL)-1-METHYLINDOLE-3-.
- EINECS
- CAS No. 110543-98-1
- Article Data11
- CAS DataBase
- Density 1.67 g/cm3
- Solubility
- Melting Point 179---180oC
- Formula C15H15Br2NO4
- Boiling Point 529.8 °C at 760 mmHg
- Molecular Weight 433.096
- Flash Point 274.2 °C
- Transport Information
- Appearance white crystalline power
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 5-acetoxy-6-bromo-2-bromomethyl-1-metyl-indol-3-ethyl carboxylate;
- PSA 57.53000
- LogP 3.93770
Synthetic route
-
-
40945-79-7
1,2-dimethyl-5-acetoxy-1H-indole-3-carboxylic acid ethyl ester

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane for 16h; Inert atmosphere; Reflux; | 99% |
| With bromine In tetrachloromethane for 2h; Heating; | 87% |
| With bromine; dibenzoyl peroxide In chloroform for 3h; Reagent/catalyst; Reflux; | 85% |
-
-
40945-79-7
1,2-dimethyl-5-acetoxy-1H-indole-3-carboxylic acid ethyl ester

-
A
-
1312943-26-2
5-acetoxy-6,7-dibromo-2-bromomethyl-1-methyl-1H-indole-3-carboxylic acid ethyl ester

-
B
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With bromine In 1,2-dichloro-ethane at 85℃; for 4h; | A 28% B 20 %Chromat. |
| With bromine In 1,2-dichloro-ethane at 85℃; for 3h; | A 23 %Chromat. B 53 %Chromat. |
-
-
15574-49-9
mecarbinate

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine 2: bromine / tetrachloromethane / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: sodium acetate / dichloromethane / 3 h / Reflux 2: hydrogen bromide; dihydrogen peroxide / 1,2-dichloro-ethane / 4.5 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: 4 h / Reflux 2: [RhCl2(p-cymene)]2; N-Bromosuccinimide / water; N,N-dimethyl acetamide / 24 h / 90 °C / Inert atmosphere View Scheme |

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate; palladium diacetate; copper diacetate / N,N-dimethyl-formamide / 3 h / 80 °C 2: potassium carbonate / N,N-dimethyl-formamide / 6 h / 100 °C 3: dibenzoyl peroxide; bromine / chloroform / 3 h / Reflux View Scheme |
-
-
20862-91-3
ethyl 5-acetyloxy-2-methyl-1H-indole-3-carboxylate

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 6 h / 100 °C 2: dibenzoyl peroxide; bromine / chloroform / 3 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydride / N,N-dimethyl-formamide / 1.5 h / Inert atmosphere; Cooling with ice 2: bromine / tetrachloromethane / 16 h / Inert atmosphere; Reflux View Scheme |
-
-
100-02-7
4-nitro-phenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: pyridine / acetone / 4 h / 20 °C / Reflux 2: iron; ammonium chloride / ethanol; water / 2 h / Reflux 3: indium(III) bromide / dichloromethane / 3 h / Reflux 4: potassium carbonate; palladium diacetate; copper diacetate / N,N-dimethyl-formamide / 3 h / 80 °C 5: potassium carbonate / N,N-dimethyl-formamide / 6 h / 100 °C 6: dibenzoyl peroxide; bromine / chloroform / 3 h / Reflux View Scheme |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
108-98-5
thiophenol

-
-
131707-24-9
6-bromo-5-hydroxy-1-methyl-2-phenylthiomethylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 3h; Ambient temperature; | 96.4% |
| Stage #1: thiophenol With sodium hydroxide In methanol for 2h; Stage #2: 5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester In methanol for 3h; | 90.8% |
| Stage #1: 5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester; thiophenol With sodium hydroxide In methanol at 15 - 20℃; for 1h; Stage #2: With acetic acid In methanol; acetone for 1h; Reflux; | 88.6% |

| Conditions | Yield |
|---|---|
| In benzene for 22h; Ambient temperature; | 91.9% |
-
-
100-02-7
4-nitro-phenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
135980-83-5
5-Acetoxy-6-bromo-1-methyl-2-(4-nitro-phenoxymethyl)-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone for 5h; Heating; | 79.2% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 0.5h; Ambient temperature; | 77.1% |
-
-
106-47-8
4-chloro-aniline

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
132629-02-8
5-Acetoxy-6-bromo-2-[(4-chloro-phenylamino)-methyl]-1-methyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 20℃; | 76.8% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
108-95-2
phenol

-
A
-
135980-84-6
5-Acetoxy-6-bromo-1-methyl-2-phenoxymethyl-1H-indole-3-carboxylic acid ethyl ester

-
B
-
135980-77-7
1-methyl-2-phenoxy-3-ethoxycarbonyl-5-(1-methyl-3-ethoxycarbonyl-5-acetoxy-6-bromoindolyl-2-methoxy)-6-bromoindole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone for 5h; Heating; | A 72.5% B 4.1% |

-
-
104-94-9
4-methoxy-aniline

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
132629-01-7
5-Acetoxy-6-bromo-2-[(4-methoxy-phenylamino)-methyl]-1-methyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 20℃; | 71.1% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
62-53-3
aniline

-
A
-
132629-00-6
5-Acetoxy-6-bromo-1-methyl-2-phenylaminomethyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 20℃; | A 69.1% B 3.2% |

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
150-76-5
4-methoxy-phenol

-
-
135980-85-7
5-Acetoxy-6-bromo-2-(4-methoxy-phenoxymethyl)-1-methyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone for 5h; Heating; | 68.3% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
100-61-8
N-methylaniline

-
-
132628-99-0
5-Acetoxy-6-bromo-1-methyl-2-[(methyl-phenyl-amino)-methyl]-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 20℃; | 65% |
-
-
106-41-2
4-bromo-phenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
135980-82-4
5-Acetoxy-6-bromo-2-(4-bromo-phenoxymethyl)-1-methyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone for 5h; Heating; | 64.5% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
17356-08-0
thiourea

| Conditions | Yield |
|---|---|
| In ethanol for 24h; Heating; | 64.2% |
-
-
22948-02-3
3-aminothiophenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 3-aminothiophenol With potassium hydroxide In methanol at 20℃; for 0.25h; Inert atmosphere; Stage #2: 5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester In methanol; dichloromethane for 3h; Inert atmosphere; Cooling with ice; | 62% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
100-01-6
4-nitro-aniline

-
-
132629-03-9
5-Acetoxy-6-bromo-1-methyl-2-[(4-nitro-phenylamino)-methyl]-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 20℃; | 60.1% |
-
-
40945-79-7
1,2-dimethyl-5-acetoxy-1H-indole-3-carboxylic acid ethyl ester

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane for 16h; Inert atmosphere; Reflux; | 99% |
| With bromine In tetrachloromethane for 2h; Heating; | 87% |
| With bromine; dibenzoyl peroxide In chloroform for 3h; Reagent/catalyst; Reflux; | 85% |
-
-
40945-79-7
1,2-dimethyl-5-acetoxy-1H-indole-3-carboxylic acid ethyl ester

-
A
-
1312943-26-2
5-acetoxy-6,7-dibromo-2-bromomethyl-1-methyl-1H-indole-3-carboxylic acid ethyl ester

-
B
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With bromine In 1,2-dichloro-ethane at 85℃; for 4h; | A 28% B 20 %Chromat. |
| With bromine In 1,2-dichloro-ethane at 85℃; for 3h; | A 23 %Chromat. B 53 %Chromat. |
-
-
15574-49-9
mecarbinate

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine 2: bromine / tetrachloromethane / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: sodium acetate / dichloromethane / 3 h / Reflux 2: hydrogen bromide; dihydrogen peroxide / 1,2-dichloro-ethane / 4.5 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: 4 h / Reflux 2: [RhCl2(p-cymene)]2; N-Bromosuccinimide / water; N,N-dimethyl acetamide / 24 h / 90 °C / Inert atmosphere View Scheme |

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate; palladium diacetate; copper diacetate / N,N-dimethyl-formamide / 3 h / 80 °C 2: potassium carbonate / N,N-dimethyl-formamide / 6 h / 100 °C 3: dibenzoyl peroxide; bromine / chloroform / 3 h / Reflux View Scheme |
-
-
20862-91-3
ethyl 5-acetyloxy-2-methyl-1H-indole-3-carboxylate

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 6 h / 100 °C 2: dibenzoyl peroxide; bromine / chloroform / 3 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydride / N,N-dimethyl-formamide / 1.5 h / Inert atmosphere; Cooling with ice 2: bromine / tetrachloromethane / 16 h / Inert atmosphere; Reflux View Scheme |
-
-
100-02-7
4-nitro-phenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: pyridine / acetone / 4 h / 20 °C / Reflux 2: iron; ammonium chloride / ethanol; water / 2 h / Reflux 3: indium(III) bromide / dichloromethane / 3 h / Reflux 4: potassium carbonate; palladium diacetate; copper diacetate / N,N-dimethyl-formamide / 3 h / 80 °C 5: potassium carbonate / N,N-dimethyl-formamide / 6 h / 100 °C 6: dibenzoyl peroxide; bromine / chloroform / 3 h / Reflux View Scheme |
-
-
830-03-5
4-nitrophenol acetate

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: iron; ammonium chloride / ethanol; water / 2 h / Reflux 2: indium(III) bromide / dichloromethane / 3 h / Reflux 3: potassium carbonate; palladium diacetate; copper diacetate / N,N-dimethyl-formamide / 3 h / 80 °C 4: potassium carbonate / N,N-dimethyl-formamide / 6 h / 100 °C 5: dibenzoyl peroxide; bromine / chloroform / 3 h / Reflux View Scheme |
-
-
13871-68-6
p-aminophenyl acetate

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: indium(III) bromide / dichloromethane / 3 h / Reflux 2: potassium carbonate; palladium diacetate; copper diacetate / N,N-dimethyl-formamide / 3 h / 80 °C 3: potassium carbonate / N,N-dimethyl-formamide / 6 h / 100 °C 4: dibenzoyl peroxide; bromine / chloroform / 3 h / Reflux View Scheme |
-
-
870-85-9
ethyl 3-methylaminocrotonate

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1,2-dichloro-ethane / 8 h / Reflux 2: pyridine / acetone / 4 h / 30 °C 3: dibenzoyl peroxide; bromine / tetrachloromethane / 5 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 2: bromine View Scheme |
-
-
7598-91-6
ethyl 5-hydroxy-2-methylindole-3-carboxylate

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: pyridine / 1 h / Inert atmosphere; Reflux 2: sodium hydride / N,N-dimethyl-formamide / 1.5 h / Inert atmosphere; Cooling with ice 3: bromine / tetrachloromethane / 16 h / Inert atmosphere; Reflux View Scheme |
-
-
141-97-9
ethyl acetoacetate

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydroxide 3: bromine View Scheme | |
| Multi-step reaction with 4 steps 1: 3 h / 20 - 30 °C / Cooling with ice 2: zinc(II) chloride / dichloromethane / 4 h / 10 - 20 °C 3: sodium acetate / dichloromethane / 3 h / Reflux 4: hydrogen bromide; dihydrogen peroxide / 1,2-dichloro-ethane / 4.5 h / Reflux View Scheme |
-
-
65578-36-1
ethyl N-methyl β-aminocrotonate

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: zinc(II) chloride / dichloromethane / 4 h / 10 - 20 °C 2: sodium acetate / dichloromethane / 3 h / Reflux 3: hydrogen bromide; dihydrogen peroxide / 1,2-dichloro-ethane / 4.5 h / Reflux View Scheme |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
108-98-5
thiophenol

-
-
131707-24-9
6-bromo-5-hydroxy-1-methyl-2-phenylthiomethylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 3h; Ambient temperature; | 96.4% |
| Stage #1: thiophenol With sodium hydroxide In methanol for 2h; Stage #2: 5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester In methanol for 3h; | 90.8% |
| Stage #1: 5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester; thiophenol With sodium hydroxide In methanol at 15 - 20℃; for 1h; Stage #2: With acetic acid In methanol; acetone for 1h; Reflux; | 88.6% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
111-92-2
dibutylamine

-
-
110566-59-1
1-Methyl-2-dibutylaminomethyl-3-ethoxycarbonyl-5-acetoxy-6-bromoindole

| Conditions | Yield |
|---|---|
| In benzene for 22h; Ambient temperature; | 91.9% |
-
-
100-02-7
4-nitro-phenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
135980-83-5
5-Acetoxy-6-bromo-1-methyl-2-(4-nitro-phenoxymethyl)-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone for 5h; Heating; | 79.2% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 0.5h; Ambient temperature; | 77.1% |
-
-
106-47-8
4-chloro-aniline

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
132629-02-8
5-Acetoxy-6-bromo-2-[(4-chloro-phenylamino)-methyl]-1-methyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 20℃; | 76.8% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
108-95-2
phenol

-
A
-
135980-84-6
5-Acetoxy-6-bromo-1-methyl-2-phenoxymethyl-1H-indole-3-carboxylic acid ethyl ester

-
B
-
135980-77-7
1-methyl-2-phenoxy-3-ethoxycarbonyl-5-(1-methyl-3-ethoxycarbonyl-5-acetoxy-6-bromoindolyl-2-methoxy)-6-bromoindole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone for 5h; Heating; | A 72.5% B 4.1% |

-
-
104-94-9
4-methoxy-aniline

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
132629-01-7
5-Acetoxy-6-bromo-2-[(4-methoxy-phenylamino)-methyl]-1-methyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 20℃; | 71.1% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
62-53-3
aniline

-
A
-
132629-00-6
5-Acetoxy-6-bromo-1-methyl-2-phenylaminomethyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 20℃; | A 69.1% B 3.2% |

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
150-76-5
4-methoxy-phenol

-
-
135980-85-7
5-Acetoxy-6-bromo-2-(4-methoxy-phenoxymethyl)-1-methyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone for 5h; Heating; | 68.3% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
100-61-8
N-methylaniline

-
-
132628-99-0
5-Acetoxy-6-bromo-1-methyl-2-[(methyl-phenyl-amino)-methyl]-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 20℃; | 65% |
-
-
106-41-2
4-bromo-phenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
135980-82-4
5-Acetoxy-6-bromo-2-(4-bromo-phenoxymethyl)-1-methyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone for 5h; Heating; | 64.5% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
17356-08-0
thiourea

| Conditions | Yield |
|---|---|
| In ethanol for 24h; Heating; | 64.2% |
-
-
22948-02-3
3-aminothiophenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 3-aminothiophenol With potassium hydroxide In methanol at 20℃; for 0.25h; Inert atmosphere; Stage #2: 5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester In methanol; dichloromethane for 3h; Inert atmosphere; Cooling with ice; | 62% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
100-01-6
4-nitro-aniline

-
-
132629-03-9
5-Acetoxy-6-bromo-1-methyl-2-[(4-nitro-phenylamino)-methyl]-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 20℃; | 60.1% |
-
-
15570-12-4
3-methoxybenzenethiol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium carbonate In ethyl acetate at 50℃; for 2h; Inert atmosphere; | 59% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
91-60-1
2-Naphthalenethiol

| Conditions | Yield |
|---|---|
| Stage #1: 2-Naphthalenethiol With potassium hydroxide In methanol at 20℃; for 0.25h; Inert atmosphere; Stage #2: 5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester In methanol; dichloromethane for 3h; Inert atmosphere; Cooling with ice; | 50% |
-
-
40248-84-8
3-mercaptophenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium carbonate In ethyl acetate at 100℃; for 5h; Inert atmosphere; | 44% |
-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
1312943-26-2
5-acetoxy-6,7-dibromo-2-bromomethyl-1-methyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With bromine In 1,2-dichloro-ethane at 85℃; for 4.5h; | 28% |
-
-
1121-24-0
ortho-mercaptophenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: ortho-mercaptophenol; 5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester With sodium carbonate In ethyl acetate at 50℃; for 2h; Inert atmosphere; Stage #2: With methanol; potassium hydroxide at 20℃; for 3h; Inert atmosphere; | 18% |
-
-
142-04-1
aniline hydrochloride

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In water for 3h; Heating; | 10.6% |
-
-
637-89-8
4-sulfanylphenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 4-sulfanylphenol; 5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester With sodium carbonate In ethyl acetate at 50℃; for 2h; Inert atmosphere; Stage #2: With methanol; potassium hydroxide at 20℃; for 3h; Inert atmosphere; | 2% |
-
-
110-91-8
morpholine

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

-
-
110543-92-5
5-Acetoxy-6-bromo-1-methyl-2-morpholin-4-ylmethyl-1H-indole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In benzene for 22h; Ambient temperature; | |
| In benzene for 1h; |
-
-
7774-74-5
Thiophene-2-thiol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide 1.) MeOH, RT, 10 min, 2.) MeOH, RT, 3 h; Yield given. Multistep reaction; |
-
-
7774-73-4
thiophene-3-thiol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide 1.) MeOH, RT, 10 min, 2.) MeOH, RT, 3 h; Yield given. Multistep reaction; |
-
-
2637-34-5
2-Mercaptopyridine

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide 1.) MeOH, RT, 10 min, 2.) MeOH, RT, 3 h; Yield given. Multistep reaction; |
-
-
1569-69-3
Cyclohexanethiol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide 1.) MeOH, RT, 10 min, 2.) MeOH, RT, 3 h; Yield given. Multistep reaction; |
-
-
950-59-4
4-hydroxy-3,5-di-tert-butylthiophenol

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide 1.) MeOH, RT, 10 min, 2.) MeOH, RT, 3 h; Yield given. Multistep reaction; |
-
-
54585-47-6
3-cyano-4,6-dimethyl-2-mercaptopyridine

-
-
110543-98-1
5-acetoxy-6-bromo-2-bromomethyl-1-methylindole-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide 1.) MeOH, RT, 10 min, 2.) MeOH, RT, 3 h; Yield given. Multistep reaction; |
Ethyl 5-acetoxy-6-bromo-2-(bromomethyl)-1-methyl-1H-indole-3-carboxylate Chemical Properties
Molecular structure of Ethyl 5-acetoxy-6-bromo-2-(bromomethyl)-1-methyl-1H-indole-3-carboxylate (CAS NO.110543-98-1) is:
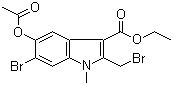
Product Name: Ethyl 5-acetoxy-6-bromo-2-(bromomethyl)-1-methyl-1H-indole-3-carboxylate
CAS Registry Number: 110543-98-1
IUPAC Name: ethyl 5-acetyloxy-6-bromo-2-(bromomethyl)-1-methylindole-3-carboxylate
Empirical Formula: C15H15Br2NO4
Molecular Weight: 433.0919
XLogP3-AA: 3.3
H-Bond Donor: 0
H-Bond Acceptor: 4
Surface Tension: 46.1 dyne/cm
Density: 1.67 g/cm3
Flash Point: 274.2 °C
Enthalpy of Vaporization: 80.49 kJ/mol
Boiling Point: 529.8 °C at 760 mmHg
Vapour Pressure: 2.62E-11 mmHg at 25°C
Product Categories: arbidol
Ethyl 5-acetoxy-6-bromo-2-(bromomethyl)-1-methyl-1H-indole-3-carboxylate Specification
Ethyl 5-acetoxy-6-bromo-2-(bromomethyl)-1-methyl-1H-indole-3-carboxylate , its cas register number is 110543-98-1. It also can be called 5-acetoxy-6-bromo-2-bromomethyl-1-metyl-indol-3-ethyl carboxylate .
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 110545-39-6
- 110545-67-0
- 110549-07-0
- 110553-27-0
- 110556-33-7
- 11055-93-9
- 11056-06-7
- 110-56-5
- 1105662-87-0
- 1105662-99-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View