-
Name
Fenbendazole
- EINECS 256-145-7
- CAS No. 43210-67-9
- Article Data13
- CAS DataBase
- Density 1.4 g/cm3
- Solubility Insoluble in water
- Melting Point 233 °C
- Formula C15H13N3O2S
- Boiling Point
- Molecular Weight 299.353
- Flash Point
- Transport Information
- Appearance Off-white solid
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Carbamicacid, [5-(phenylthio)-1H-benzimidazol-2-yl]-, methyl ester (9CI);2-(Methoxycarbonylamino)-5-(phenylthio)benzimidazole;Axilur;Fenbendazol;Fenbion;HOE 881;Methyl5-(phenylthio)-2-benzimidazolecarbamate;Methyl[5-(phenylthio)-1H-benzimidazol-2-yl]carbamate;Panacur;Safe-Guard;
- PSA 92.31000
- LogP 3.96540
Synthetic route
-
-
43156-48-5
1,2-diamino-4-phenylthiobenzene

-
-
867-44-7
S-Methylisothiourea sulfate

-
-
79-22-1
methyl chloroformate

-
-
43210-67-9
Fenbendazole

| Conditions | Yield |
|---|---|
| Stage #1: S-Methylisothiourea sulfate; methyl chloroformate With sodium hydroxide In water at 3 - 6℃; for 0.666667h; Stage #2: 1,2-diamino-4-phenylthiobenzene With acetic acid In ethanol; water at 95℃; for 24h; | 88% |

-
-
43156-47-4
2-nitro-5-phenylthioaniline

-
-
43210-67-9
Fenbendazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: H2 / Pd/C / ethanol / 20 °C / 3102.89 Torr 2.1: NaOH / H2O / 0.67 h / 3 - 6 °C 2.2: 88 percent / AcOH / H2O; ethanol / 24 h / 95 °C View Scheme |
-
-
1635-61-6
5-chloro-2-nitroaniline

-
-
43210-67-9
Fenbendazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: K2CO3 / dimethylformamide / 6 h / Heating 2.1: H2 / Pd/C / ethanol / 20 °C / 3102.89 Torr 3.1: NaOH / H2O / 0.67 h / 3 - 6 °C 3.2: 88 percent / AcOH / H2O; ethanol / 24 h / 95 °C View Scheme |
-
-
108-98-5
thiophenol

-
-
43210-67-9
Fenbendazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: K2CO3 / dimethylformamide / 6 h / Heating 2.1: H2 / Pd/C / ethanol / 20 °C / 3102.89 Torr 3.1: NaOH / H2O / 0.67 h / 3 - 6 °C 3.2: 88 percent / AcOH / H2O; ethanol / 24 h / 95 °C View Scheme |
- (5-phenylsulfanyl-1-phosphonooxymethyl-1H-benzoimidazol-2-yl)carbamic acid methyl ester disodium salt; (6-phenylsulfanyl-1-phosphonooxymethyl-1H-benzoimidazol-2-yl)carbamic acid methyl ester disodium salt; mixture of
-
(5-phenylsulfanyl-1-phosphonooxymethyl-1H-benzoimidazol-2-yl)carbamic acid methyl ester disodium salt; (6-phenylsulfanyl-1-phosphonooxymethyl-1H-benzoimidazol-2-yl)carbamic acid methyl ester disodium salt; mixture of

-
-
43210-67-9
Fenbendazole

| Conditions | Yield |
|---|---|
| With chicken alkaline phosphatase In water at 39℃; pH=6.5 - 9.0; Enzyme kinetics; | |
| With porcine alkaline phosphatase In water at 37℃; pH=6.5 - 9.0; Enzyme kinetics; |
-
-
43210-67-9
Fenbendazole

-
-
53716-50-0, 122063-22-3, 122063-23-4
oxfendazole

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; sodium sulfite In methanol; water | 98.8% |
| With urea hydrogen peroxide adduct In formic acid; water at 25 - 45℃; for 5.5h; Temperature; Concentration; | 98% |
-
-
43210-67-9
Fenbendazole

-
-
53065-28-4
5-(phenylthio)-1H-benzo[d]imidazol-2-amine

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water for 48h; Heating; | 98% |
| In N,N-dimethyl-formamide for 12h; Heating; | 50% |
-
-
541-41-3
chloroformic acid ethyl ester

-
-
43210-67-9
Fenbendazole

-
A
-
58521-88-3
2-Methoxycarbonylamino-5-phenylsulfanyl-benzoimidazole-1-carboxylic acid ethyl ester

-
B
-
58522-05-7
2-Methoxycarbonylamino-6-phenylsulfanyl-benzoimidazole-1-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane Ambient temperature; |

-
-
79-22-1
methyl chloroformate

-
-
43210-67-9
Fenbendazole

-
A
-
58521-87-2
methyl 1-methoxycarbonyl-5-phenylthiobenzimidazole-2-carbamate

-
B
-
58522-04-6
2-Methoxycarbonylamino-6-phenylsulfanyl-benzoimidazole-1-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane for 20h; Ambient temperature; | A 12 g B 8 g |

-
-
43210-67-9
Fenbendazole

-
-
2941-64-2
Ethyl chlorothioformate

-
A
-
104663-11-8
2-Methoxycarbonylamino-5-phenylsulfanyl-benzoimidazole-1-carbothioic acid S-ethyl ester

-
B
-
104663-33-4
2-Oxo-5-phenylsulfanyl-benzoimidazole-1,3-dicarbothioic acid di-S-ethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane Ambient temperature; |

-
-
43210-67-9
Fenbendazole

-
-
74-88-4
methyl iodide

| Conditions | Yield |
|---|---|
| With AG-MP1 anion exchange resin In carbon dioxide at 80℃; under 150012 Torr; for 0.333333h; Product distribution; further conditions: CH3CN, room temperature, 1 h; |
-
-
43210-67-9
Fenbendazole

-
-
104663-22-1
6-Benzenesulfinyl-2-methoxycarbonylamino-benzoimidazole-1-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 8 g / 1N aq sodium hydroxide / CH2Cl2 / 20 h / Ambient temperature 2: m-chloroperbenzoic acid / CH2Cl2 View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
104663-01-6
1-carbomethoxy-2-carbomethoxyamino-5-phenylsulfinylbenzimidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 12 g / 1N aq sodium hydroxide / CH2Cl2 / 20 h / Ambient temperature 2: 8 g / m-chloroperbenzoic acid / CH2Cl2 / 16 h / 15 - 20 °C View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
104663-08-3
1-carbomethoxy-2-carbomethoxyamino-5-phenylsulfonylbenzimidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 12 g / 1N aq sodium hydroxide / CH2Cl2 / 20 h / Ambient temperature 2: 1.5 g / m-chloroperbenzoic acid / CH2Cl2 View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
104663-29-8
6-Benzenesulfonyl-2-methoxycarbonylamino-benzoimidazole-1-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 8 g / 1N aq sodium hydroxide / CH2Cl2 / 20 h / Ambient temperature 2: m-chloroperbenzoic acid / CH2Cl2 View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
104663-02-7
5-Benzenesulfinyl-2-methoxycarbonylamino-benzoimidazole-1-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1N aq sodium hydroxide / CH2Cl2 / Ambient temperature 2: m-chloroperbenzoic acid / CH2Cl2 View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
104663-12-9
5-Benzenesulfinyl-2-methoxycarbonylamino-benzoimidazole-1-carbothioic acid S-ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1N aq sodium hydroxide / CH2Cl2 / Ambient temperature 2: m-chloroperbenzoic acid / CH2Cl2 View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
104663-23-2
6-Benzenesulfinyl-2-methoxycarbonylamino-benzoimidazole-1-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1N aq sodium hydroxide / CH2Cl2 / Ambient temperature 2: m-chloroperbenzoic acid / CH2Cl2 View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
59530-20-0
2-amino-5(6)-benzenesulfonylbenzimidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 82 percent / pyridine / 1 h / Heating 3: 82.6 percent / KMnO4 / acetic acid / 2 h / Ambient temperature 4: 73.5 percent / aq. NaOH / ethanol / 2 h / Ambient temperature View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
125422-34-6
2-Acetamido-5(6)-phenylthiobenzimidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 82 percent / pyridine / 1 h / Heating View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
125422-38-0
5(6)-Phenylthio-2-(carboxamidomethylamino)-benzimidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 50 percent / triethylamine / acetone / 12 h / Heating View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
125422-41-5
5(6)-Phenylthio-2-chloroacetamidobenzimidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 88 percent / triethylamine / acetone / 3 h / Ambient temperature View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
125422-35-7
2-Acetamido-5(6)-phenylsulphonobenzimidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 82 percent / pyridine / 1 h / Heating 3: 82.6 percent / KMnO4 / acetic acid / 2 h / Ambient temperature View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
125422-37-9
(5-Phenylsulfanyl-1H-benzoimidazol-2-ylamino)-acetic acid methyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 64 percent / triethylamine View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
125443-69-8
2-(Carbethoxymethylamino)-5(6)-phenylthiobenzimidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 55 percent / triethylamine / acetone / 12 h / Heating View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
125443-68-7
2,2,2-Trifluoro-N-(5-phenylsulfanyl-1H-benzoimidazol-2-yl)-acetamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 71 percent / 3 h / Heating View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
125422-49-3
2-Chloroacetamido-5(6)-phenylsulphonobenzimidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 82 percent / pyridine / 1 h / Heating 3: 82.6 percent / KMnO4 / acetic acid / 2 h / Ambient temperature 4: 73.5 percent / aq. NaOH / ethanol / 2 h / Ambient temperature 5: 80 percent / triethylamine / tetrahydrofuran / 3 h / Ambient temperature View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
125422-39-1
2-(5-Phenylsulfanyl-1H-benzoimidazol-2-ylamino)-1-piperidin-1-yl-ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 56 percent / triethylamine View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
125422-42-6
2-(1-Pyrrolidinoacetylamino)-5(6)-phenylthiobenzimidazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 88 percent / triethylamine / acetone / 3 h / Ambient temperature 3: 64 percent / triethylamine / acetone / 12 h / Heating View Scheme |
-
-
43210-67-9
Fenbendazole

-
-
125422-43-7
N-(5-Phenylsulfanyl-1H-benzoimidazol-2-yl)-2-piperidin-1-yl-acetamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 98 percent / KOH / methanol; H2O / 48 h / Heating 2: 88 percent / triethylamine / acetone / 3 h / Ambient temperature 3: 67 percent / triethylamine View Scheme |
FENBENDAZOLE Chemical Properties
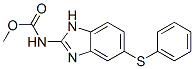
IUPAC:methyl N-(6-phenylsulfanyl-1H-benzimidazol-2-yl)carbamate
CAS:43210-67-9
The Molecular formula of Fenbendazole(43210-67-9):C15H13N3O2S
The Molecular Weight of Fenbendazole(43210-67-9):299.35
Synonyms:[5-(PHENYLTHIO)-1H-BENZIMIDAZOL-2-YL]CARBAMIC ACID, METHYL ESTER;HOE-881V;FENBENDAZOLE;METHYL 5-(PHENYLTHIO)-2-BENZIMIDAZOLECARBAMATE;SAFEGARD;PANACUR;(5-(phenylthio)-1h-benzimidazol-2-yl)-carbamicacimethylester;5-(phenylthio)-2-benzimidazolecarbamicacimethylester
EINECS:256-145-7
Density:1.4g/cm3
Melting Point:233oC
Appearance:white to yellowish powder
Product Categories:PHARMACEUTICALS;Active Pharmaceutical Ingredients;API;Intermediates & Fine Chemicals;Veterinaries
Mol File:43210-67-9.mol
storage temp.:0-6oC
FENBENDAZOLE Uses
Fenbendazole(43210-67-9) is known as a kind of benzimidazole anthelmintics. It is regularly used to eliminate numerous gastrointestinal parasites from animals,and it is quite effective against roundworms, hookworms, whipworms, certain tapeworms and parasites called strongyles and strongyloides.
FENBENDAZOLE Toxicity Data With Reference
| 1. | oth:hmn:lym 100 mg/L | ENMUDM Environmental Mutagenesis. 2 (1980),67. | ||
| 2. | oth:hmn:leu 1 mg/L | THERAP Therapie. 31 (1976),505. |
FENBENDAZOLE Safety Profile
Human mutation data reported. When heated to decomposition it emits toxic fumes of SOx and NOx.
Safty informations about Fenbendazole(43210-67-9):
Hazard Codes:Xi
Risk Statements:36/37/38
36/37/38(Irritating to eyes, respiratory system and skin)
Safety Statements:26-36
26(In case of contact with eyes, rinse immediately with plenty of water and seek medical advice)
36(Wear suitable protective clothing)
WGK Germany:2
RTECS:DD6520500
FENBENDAZOLE Specification
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View