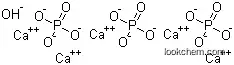-
Name
Hydroxyapatite
- EINECS 215-145-7
- CAS No. 1306-06-5
- Density 3.076 g/cm3(Temp: 18 °C)
- Solubility water: 0.3 mg/mL
- Melting Point 1100 °C(lit.)
- Formula Ca5.(OH).(PO4)3
- Boiling Point 158oC at 760 mmHg
- Molecular Weight 502.31
- Flash Point
- Transport Information
- Appearance white powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Tricalcium Phosphate (TCP);Mono Calcium Phosphate(MCP);Hy-Apatite;Tri-Tab;Hyaluronic acid(HA);Apaceram;Interpore 500;Interpore 200;Supertite 10;HAP-B;Monite;
- PSA 119.12000
- LogP 0.20920
Hydroxyapatite Consensus Reports
Hydroxyapatite Specification
The IUPAC name of Hydroxyapatite is pentacalcium hydroxide triphosphate. With the CAS registry number 1306-06-5, it is also named as Durapatite. The product's category is Inorganics , and the other registry numbers are 1059174-00-3; 12440-80-1; 136841-77-5; 196875-13-5. Besides, it is white powder, which should be stored in a cool, ventilated, dry place at -20 °C. In addition, its molecular formula is Ca5.(OH).(PO4)3 and molecular weight is 502.31.It is the mineral component of bones and teeth; it has been used therapeutically as a prosthetic aid and in the prevention and treatment of osteoporosis.
The other characteristics of Hydroxyapatite can be summarized as: (1)H-Bond Donor: 1; (2)H-Bond Acceptor: 13; (3)Rotatable Bond Count: 0; (4)Exact Mass: 501.675956; (5)MonoIsotopic Mass: 501.675956; (6)Topological Polar Surface Area: 260; (7)Heavy Atom Count: 21; (8)Complexity: 36.8; (9)EINECS: 215-145-7; (10)Melting Point: 1100 °C; (11)Solubility: H2O: 0.3 mg/mL.
Preparation of Hydroxyapatite: First, please wash and defecate the fresh bone. And then steam it to remove osseocolla and fat. At last, you would obtain this chemical by drying and crushing.
Uses of Hydroxyapatite: Hydroxyapatite is used to replace amputated bone as a filler or to promote bone ingrowth into prosthetic implants as a coating. And it is a calcium supplement derived from bovine bone to encourage natural growth of skin around it. Moreover, it can be analysed in order to reconstruct ancient diets in archaeology.
When you are using Hydroxyapatite , please be cautious about it as the following: it is irritating to eyes, respiratory system and skin. In case of contact with eyes, please rinse immediately with plenty of water and seek medical advice. And you should wear suitable protective clothing.
You can still convert the following datas into molecular structure:
(1)SMILES: [OH-].[O-]P(=O)([O-])[O-].[O-]P(=O)([O-])[O-].[O-]P(=O)([O-])[O-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2]
(2)InChI: InChI=1/5Ca.3H3O4P.H2O/c;;;;;3*1-5(2,3)4;/h;;;;;3*(H3,1,2,3,4);1H2/q5*+2;;;;/p-10
(3)InChIKey: XYJRXVWERLGGKC-RDRJDVFWAH
The toxicity data of Hydroxyapatite is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | oral | > 30gm/kg (30000mg/kg) | Drugs in Japan Vol. -, Pg. 1007, 1995. | |
| mouse | LD50 | oral | > 99500mg/kg (99500mg/kg) | Drugs in Japan Vol. -, Pg. 1007, 1995. | |
| mouse | LD50 | subcutaneous | > 25500mg/kg (25500mg/kg) | Drugs in Japan Vol. -, Pg. 1007, 1995. | |
| rat | LD50 | oral | > 25350mg/kg (25350mg/kg) | Drugs in Japan Vol. -, Pg. 1007, 1995. | |
| rat | LD50 | subcutaneous | > 19850mg/kg (19850mg/kg) | Drugs in Japan Vol. -, Pg. 1007, 1995. |
Related Products
- Hydroxyapatite
- 1306-08-7
- 130609-48-2
- 130-61-0
- 1306-19-0
- 13061-96-6
- 1306-23-6
- 130623-81-3
- 1306-24-7
- 130624-89-4
- 1306-25-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View