-
Name
Isovaleric acid
- EINECS 207-975-3
- CAS No. 503-74-2
- Article Data376
- CAS DataBase
- Density 0.963 g/cm3
- Solubility 25 g/L (20 °C) in water
- Melting Point -35 °C
- Formula C5H10O2
- Boiling Point 175.295 °C at 760 mmHg
- Molecular Weight 102.133
- Flash Point 73.402 °C
- Transport Information UN 3265 8/PG 2
- Appearance colorless to yellowish transparent liquid.
- Safety 26-36/37/39-45-38-28A
- Risk Codes 34-24-22
-
Molecular Structure
-
Hazard Symbols
 C,
C, T
T
- Synonyms Isopentanoicacid;Isovalericacid (8CI);3-Methyl-n-butyric acid;3-Methylbutanoic acid;3-Methylbutyric acid;3-Methylbutyrate;Isopropylacetic acid;b-Methylbutyric acid;Delphinic acid;Acetic acid, isopropyl-;
- PSA 37.30000
- LogP 1.11710
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium acetate; palladium diacetate; C34H36O8 at 30 - 35℃; for 6h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; | 99.1% |
| With dihydrogen peroxide In water; acetonitrile at 45℃; for 1h; chemoselective reaction; | 98% |
| With selenium(IV) oxide; dihydrogen peroxide In tetrahydrofuran for 3h; Heating; | 91% |
-
-
10323-40-7, 565-74-2
2-bromoisovaleric acid

-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| With indium; sodium dodecyl-sulfate at 20℃; for 1h; | 99% |
| With 2,2'-azobis(isobutyronitrile); hypophosphorous acid; sodium hydrogencarbonate In water for 1.5h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; α,α′-bis(2-pyridyl(tert-butyl)phosphino)-o-xylene; water; palladium(II) acetylacetonate; acetic acid at 20 - 100℃; under 30003 Torr; for 20h; Inert atmosphere; Autoclave; | 99% |
-
-
110-62-3
pentanal

-
-
96-17-3, 57456-98-1
2-Methylbutyraldehyde

-
-
590-86-3
isovaleraldehyde

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
116-53-0, 600-07-7
2-Methylbutanoic acid

-
C
-
109-52-4
valeric acid

| Conditions | Yield |
|---|---|
| With oxygen; valerianate de potassium; iron In water at 50℃; for 6h; Product distribution / selectivity; | A n/a B n/a C 98.3% |
| With oxygen; iron at 50℃; for 6h; Product distribution / selectivity; | A n/a B n/a C 97% |
| With oxygen at 50℃; for 6h; Product distribution / selectivity; | A n/a B n/a C 95% |
| With oxygen; valerianate de potassium In water at 50℃; for 6h; Product distribution / selectivity; | A n/a B n/a C 93.8% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium bromite; sodium bromide In water at 0℃; for 12h; | 95% |
| With alkaline aqueous sodium hypochlorite |
-
-
67-56-1
methanol

-
-
1201829-92-6
(R)-3-methyl-4,4-bis(phenylsulfonyl)butanoic acid

-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| With magnesium | 95% |

| Conditions | Yield |
|---|---|
| With nickel In tetrahydrofuran at 20℃; for 0.5h; Reduction; | 93% |
| With sodium tetrahydroborate; nickel dichloride In methanol; water at 20℃; for 0.5h; | 86% |
| With potassium hydroxide; hydrogen; [RhCl(Ph3P)2]; Ph2PO2CCH=CMe2 In acetone at 22℃; under 2280 Torr; for 17h; | 80% |

| Conditions | Yield |
|---|---|
| With calcium hypochlorite; acetic acid In dichloromethane; water for 2h; Ambient temperature; | 92% |

| Conditions | Yield |
|---|---|
| With oxygen; chromium; valerianate de potassium In water at 50℃; for 2.5h; Product distribution / selectivity; | A n/a B 90.3% |
| With oxygen; valerianate de potassium; copper In water at 50℃; for 2.5h; Product distribution / selectivity; | A n/a B 88.3% |
| With oxygen; sodium valerate; iron In water at 50℃; for 2.5h; Product distribution / selectivity; | A n/a B 86.2% |


| Conditions | Yield |
|---|---|
| With potassium phosphate buffer at 30℃; for 19h; Rhodococcus sp. AJ270 cells; | 90.2% |
| With benzene-1,2-dicarboxylic acid for 0.75h; microwave irradiation; | 90% |
-
-
568598-80-1
3-methylbutanoic acid 2-oxo-1,2,2-triphenylethyl ester

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
201-68-3
13-oxa-indeno[1,2-l]phenanthrene

| Conditions | Yield |
|---|---|
| With air In ethanol; acetonitrile Irradiation; | A 90% B n/a |
-
-
129571-49-9
2-(2-methylpropylidene)-1,3-dithiane 1-oxide

-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; acetonitrile at 55 - 65℃; for 11h; | 90% |

| Conditions | Yield |
|---|---|
| With poly-4-vinylpyridine In N,N-dimethyl-formamide for 0.0666667h; microwave irradiation; | 84% |
| at 180℃; |

| Conditions | Yield |
|---|---|
| With nitric acid In water at 25 - 30℃; for 4h; | 81% |
| With dihydrogen peroxide; ortho-tungstic acid In water at 90℃; for 24h; | 79% |
| With superoxide; oxygen In N,N-dimethyl-formamide for 15h; electrolysis, divided electrolytic cell, mercury pool cathode, platinum foil anode, Bu4N+Br-, cyclohexene, constant potential -1.0 V vs. SCE, current 100 (initial) to 15 (final) mA; | 62% |
-
-
3068-88-0, 32082-74-9, 36536-46-6, 65058-82-4
4-methyloxetan-2-one

-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran 1.) -30 deg C, 1 h, 2.) 0 deg C, 1 h; | 80% |
-
-
82518-38-5, 82518-48-7
(Z)-2-Dimethylamino-4-methyl-pent-2-enenitrile

-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 1h; Heating; | 79% |

| Conditions | Yield |
|---|---|
| With sodium bromate; sodium hydrogensulfite for 2h; Ambient temperature; | A 5% B 76% |
| With iodosylbenzene; potassium bromide In water for 12h; sonication; | A 86 % Chromat. B n/a |
-
-
77331-32-9
5-Isobutyl-3-methylsulfanyl-1,4-diphenyl-4H-[1,2,4]triazol-1-ium; iodide

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
74-93-1
methylthiol

-
C
-
621-12-5
1,4-diphenylsemicarbazide

| Conditions | Yield |
|---|---|
| With potassium hydroxide | A 75% B n/a C n/a |

-
-
84229-01-6
3-Methyl-2-phenylmethanesulfonyl-butyric acid

-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| With ethanol; sodium In tetrahydrofuran for 24h; Ambient temperature; | 72% |
-
-
10303-64-7
2-hydroxy-4-methylpentanoic acid

-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| With calcium hypochlorite; acetic acid In dichloromethane; water for 1h; Ambient temperature; | 70% |
-
-
108-83-8
diisobutyl ketone

-
-
100-52-7
benzaldehyde

-
A
-
503-74-2
3-methylbutyric acid

-
B
-
15325-61-8, 1608-28-2, 15325-56-1
(E)-(3-methylbut-1-en-1-yl)benzene

| Conditions | Yield |
|---|---|
| With boron trifluoride diacetate In hexane for 4h; Aldol-Grob reaction; Heating; | A 68% B n/a |


| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile at 20℃; for 4h; | 67% |
-
-
3068-88-0, 32082-74-9, 36536-46-6, 65058-82-4
4-methyloxetan-2-one

-
-
75-16-1
methylmagnesium bromide

-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| copper(l) chloride In tetrahydrofuran at 0℃; for 0.25h; | 52% |
-
-
84228-97-7
methyl 2-benzylsulfonyl-3-methylbutyrate

-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| With ethanol; sodium In tetrahydrofuran for 48h; Ambient temperature; | 52% |
-
-
64190-48-3, 65284-00-6, 70470-05-2, 1679-49-8
dihydro-4-methyl-2(3H)-furanone

-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; W(OTf)6; hydrogen at 180℃; under 760.051 Torr; for 12h; | 46% |
| With palladium on activated carbon; W(OTf)6; hydrogen In neat (no solvent) at 180℃; under 760.051 Torr; for 12h; | 46% |

| Conditions | Yield |
|---|---|
| With sodium sulfide In ethanol; water at 80℃; for 3h; | A n/a B 30% |
-
-
54129-53-2
isovaleraldehyde cyanohydrin

-
A
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| With sodium persulfate; silver nitrate In water at 60℃; for 3h; | A 22% B 9% |
| With sodium persulfate; silver nitrate In water at 60℃; for 5h; Rate constant; competition with tert-butanol; | A 22% B 9% |

| Conditions | Yield |
|---|---|
| With tetraethylammonium chloride; silver; magnesium In acetonitrile at 0℃; under 760.051 Torr; Electrolysis; | 22% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole; [bis(acetoxy)iodo]benzene In dichloromethane at 20℃; for 1h; | A 2% B 15% |
| With oxygen; copper at 260 - 270℃; | |
| With Pt#Bi2O3; oxygen In water at 90℃; under 750.075 Torr; for 5h; Autoclave; |

| Conditions | Yield |
|---|---|
| With nickel at 150℃; under 76000 Torr; Hydrogenation; |
-
-
503-74-2
3-methylbutyric acid


-
-
35413-80-0, 54676-87-8, 54676-89-0, 112346-38-0, 125711-61-7
3',4'-di-O-isovaleryl-cis-khellactone

| Conditions | Yield |
|---|---|
| With 4-pyrrolidin-1-ylpyridine; dicyclohexyl-carbodiimide In dichloromethane for 18h; Heating; | 100% |
-
-
890932-98-6
(R)-4-Benzyl-3-{(2R,3R,4R)-3-hydroxy-4-[(2R,4R,5R)-2-(4-methoxy-phenyl)-5-methyl-[1,3]dioxan-4-yl]-2-methyl-pentanoyl}-oxazolidin-2-one

-
-
503-74-2
3-methylbutyric acid

-
-
890932-99-7
3-Methyl-butyric acid (1R,2R)-3-((R)-4-benzyl-2-oxo-oxazolidin-3-yl)-1-{(S)-1-[(2R,4R,5R)-2-(4-methoxy-phenyl)-5-methyl-[1,3]dioxan-4-yl]-ethyl}-2-methyl-3-oxo-propyl ester

| Conditions | Yield |
|---|---|
| With dmap; 2,4,6-trichlorobenzoyl chloride; triethylamine In toluene at -78 - 20℃; for 1.5h; Yamaguchi esterification; | 100% |
-
-
503-74-2
3-methylbutyric acid

-
-
170918-42-0
(R)-(-)-5,5-dimethyl-4-phenyl-2-oxazolidinone

-
-
1059153-44-4
C16H21NO3

| Conditions | Yield |
|---|---|
| 100% |
-
-
503-74-2
3-methylbutyric acid

-
-
111602-93-8
(13E)-labda-8,13-diene-6β,7α,15-triol

-
-
1061674-78-9
(13E)-6β,7α,15-triisovaleryloxy-labda-8,13-diene

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylbutyric acid With 2,4,6-trichlorobenzoyl chloride; triethylamine In toluene at 20℃; for 2h; Stage #2: (13E)-labda-8,13-diene-6β,7α,15-triol In toluene at 80℃; for 2h; Further stages.; | 100% |
-
-
123-75-1
pyrrolidine

-
-
503-74-2
3-methylbutyric acid

-
-
60026-17-7
3-methyl-1-(pyrrolidin-1-yl)butan-1-one

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; for 14h; Inert atmosphere; | 100% |
-
-
503-74-2
3-methylbutyric acid

-
-
75-36-5
acetyl chloride

-
-
18667-97-5
(S)-2-acetoxy-3-methylbutanoic acid

| Conditions | Yield |
|---|---|
| at 20℃; for 2h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylbutyric acid; (1R,2S)-(+)-10,2-camphorsultam With thionyl chloride In N,N-dimethyl-formamide; toluene at 80℃; for 6h; Stage #2: With sodium carbonate In water; N,N-dimethyl-formamide; toluene Product distribution / selectivity; | 99% |
-
-
503-74-2
3-methylbutyric acid

-
-
1100106-37-3
(1R,3S,4S,5S,8R)-4-benzyloxy-8-hydroxy-3-methoxy-2,6-dioxabicyclo[3.3.0]octane

-
-
1100106-43-1
C19H26O6

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 0.5h; | 99% |
-
-
503-74-2
3-methylbutyric acid

-
-
1408075-34-2
1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-6-yl]-piperidin-4-amine

-
-
1450979-73-3
N-{1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-6-yl]piperidin-4-yl}-3-methylbutanamide

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In tetrahydrofuran for 16h; | 99% |

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromide; potassium carbonate In dichloromethane at 20℃; Molecular sieve; | 99% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine at 20℃; for 18h; | 99% |

| Conditions | Yield |
|---|---|
| With aluminum(III) sulphate octadecahydrate at 130℃; for 0.25h; Sealed tube; Microwave irradiation; | 98.1% |
| Heating; | 96% |
| With Rhizomucor miehei lipase In n-heptane at 40℃; for 24h; Enzymatic reaction; | 38.7% |
-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylbutyric acid With diphenyl phosphoryl azide; triethylamine In toluene at 90℃; Inert atmosphere; Stage #2: benzyl 2-aminoacetate hydrohloride With triethylamine In toluene at 20 - 30℃; Inert atmosphere; | 98.1% |

| Conditions | Yield |
|---|---|
| With silica gel at 130℃; for 1.33333h; Microwave irradiation; Green chemistry; | 98% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; Inert atmosphere; | 78% |
| With 1-methyl-3-(4-sulfonylbutyl)-1H-imidazol-3-ium trifluoromethanesulfonate at 96 - 100℃; for 6h; chemoselective reaction; | 52% |
| With trichlorophosphate |
-
-
503-74-2
3-methylbutyric acid

-
-
530-62-1
1,1'-carbonyldiimidazole

-
-
10364-92-8
1-(1H-imidazol-1-yl)-3-methylbutan-1-one

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 2.25h; | 98% |
| In dichloromethane for 4h; Ambient temperature; | 58% |
| In tetrahydrofuran at 20℃; for 0.5h; |
-
-
503-74-2
3-methylbutyric acid

-
-
500-99-2
3,5-dimethoxyphenol

-
-
68754-16-5
1-(2-hydroxy-4,6-dimethoxyphenyl)-3-methylbutan-1-one

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate at 80℃; for 2h; | 98% |
| With boron trifluoride diethyl etherate at 80℃; Acylation; | 84% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 23℃; for 0.5h; | 98% |
-
-
503-74-2
3-methylbutyric acid

-
-
10323-40-7, 565-74-2
2-bromoisovaleric acid

| Conditions | Yield |
|---|---|
| With bromine; phosphorus trichloride for 4.5h; Heating; | 97% |
| With PPA; bromine at 75 - 80℃; for 6h; | 94% |
| With PPA; bromine at 120℃; |
-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| In water byproducts: thorium(IV) hydroxide; dissoln. of freshly pptd. Th(IV) hydroxide in carboxylic acid; elem. anal.; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylbutyric acid; para-bromoacetophenone With trifluoroacetic anhydride In dichloromethane at 20℃; for 0.25h; Stage #2: With trifluorormethanesulfonic acid In dichloromethane at 20℃; for 9h; | 97% |

-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0℃; for 6h; | 96% |
-
-
887326-66-1
2-benzyloxycarbonylamino-3-(4-{6-(tert-butyl-dimethyl-silanyloxymethyl)-5-[6-(tert-butyl-dimethyl-silanyloxymethyl)-5-hydroxy-5,6-dihydro-2H-pyran-2-yloxy]-5,6-dihydro-2H-pyran-2-yloxy}-phenyl)-propionic acid methyl ester

-
-
503-74-2
3-methylbutyric acid

-
-
887326-67-2
3-methyl-butyric acid 6-[6-[4-(2-benzyloxycarbonylamino-2-methoxycarbonyl-ethyl)-phenoxy]-2-(tert-butyl-dimethyl-silanyloxymethyl)-3,6-dihydro-2H-pyran-3-yloxy]-2-(tert-butyl-dimethyl-silanyloxymethyl)-3,6-dihydro-2H-pyran-3-yl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0℃; | 96% |
-
-
503-74-2
3-methylbutyric acid

-
-
3053-46-1, 23086-58-0
uranium(IV) acetate

| Conditions | Yield |
|---|---|
| In not given excess of carboxylic acid, refluxing; elem. anal.; | 96% |
-
-
503-74-2
3-methylbutyric acid

-
-
41324-66-7
H-Pro-OBzl

-
-
1215172-43-2
(S)-benzyl 1-(3-methylbutanoyl)pyrrolidine-2-carboxylate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane | 96% |
-
-
503-74-2
3-methylbutyric acid

-
-
41873-65-8
ethyl 2-(2-hydroxyphenyl)acetate

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 12h; Steglich Esterification; Inert atmosphere; | 96% |
-
-
503-74-2
3-methylbutyric acid

| Conditions | Yield |
|---|---|
| With boric acid In toluene for 24h; Dean-Stark; | 96% |
Isovaleric acid Specification
The Isovaleric acid, with the CAS registry number 503-74-2, is also known as Butanoic acid, 3-methyl-. It belongs to the product categories of Pharmaceutical Raw Materials; Artemisia Vulgaris; Building Blocks; C1 to C5; Carbonyl Compounds; Carboxylic Acids; Carthamus Tinctorius (Safflower Oil); Chemical Synthesis; Humulus Lupulus (Hops); Hypericum Perforatum (St John′;Nutrition Research; Organic Building Blocks; Panax Ginseng; Phytochemicals by Plant (Food/Spice/Herb) s Wort). Its EINECS registry number is 207-975-3. This chemical's molecular formula is C5H10O2 and molecular weight is 102.13. What's more, both is IUPAC name and systematic name are the same which is called 3-Methylbutanoic acid. Isovaleric acid has a strong pungent cheesy or sweaty smell, but its volatile esters have pleasing scents and are used widely in perfumery. It has been proposed that it is the anticonvulsant agent in valerian. It is a major component of the cause of unpleasant foot odor.
Physical properties about Isovaleric acid are: (1)ACD/LogP: 1.051; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.25; (4)ACD/LogD (pH 7.4): -1.54; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 14.13; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 37.3 Å2; (13)Index of Refraction: 1.418; (14)Molar Refractivity: 26.736 cm3; (15)Molar Volume: 106.072 cm3; (16)Polarizability: 10.599×10-24cm3; (17)Surface Tension: 30.985 dyne/cm; (18)Density: 0.963 g/cm3; (19)Flash Point: 73.402 °C; (20)Enthalpy of Vaporization: 45.387 kJ/mol; (21)Boiling Point: 175.295 °C at 760 mmHg; (22)Vapour Pressure: 0.554 mmHg at 25 °C.
Preparation of Isovaleric acid: this chemical can be prepared by 3-methyl-butan-1-ol. This reaction needs reagent superoxide/oxygen and solvent dimethylformamide. The reaction time is 15 hours. The yield is 62 %.
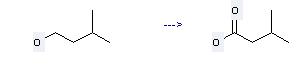
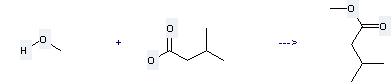
Uses of Isovaleric acid: it is used to produce other chemicals. For example, it can react with methanol to get 4-methyl-butyric acid methyl ester. The reaction occurs with reagent H2SO4 and solvent CH2Cl2.
When you are dealing with this chemical, you should be very careful. This chemical may destroy living tissue on contact and may cause damage to health at low levels. It is harmful if swallowed, toxic in contact with skin and may cause burns. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice. In case of insufficient ventilation you must wear suitable respiratory equipment. And in case of accident or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(O)CC(C)C
(2) InChI: InChI=1S/C5H10O2/c1-4(2)3-5(6)7/h4H,3H2,1-2H3,(H,6,7)
(3) InChIKey: GWYFCOCPABKNJV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 1120mg/kg (1120mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Acta Pharmacologica et Toxicologica. Vol. 18, Pg. 141, 1961. |
| rabbit | LD50 | skin | 310uL/kg (.31mL/kg) | Union Carbide Data Sheet. Vol. 1/31/1972, | |
| rat | LD50 | oral | 2mL/kg (2mL/kg) | Union Carbide Data Sheet. Vol. 1/31/1972, |
Related Products
- Isovaleric acid
- ISOVALERIC ACID, ALLYL ESTER
- 50375-10-5
- 50376-99-3
- 50377-49-6
- 50381-53-8
- 50382-14-4
- 50382-32-6
- 503-83-3
- 503859-26-5
- 503859-27-6
- 503-87-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View