-
Name
L-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid
- EINECS -0
- CAS No. 74163-81-8
- Article Data35
- CAS DataBase
- Density 1.225 g/cm3
- Solubility
- Melting Point 300 °C
- Formula C10H11NO2
- Boiling Point 372 °C at 760 mmHg
- Molecular Weight 177.203
- Flash Point 178.8 °C
- Transport Information
- Appearance white to light yellow crystal powder
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 3-Isoquinolinecarboxylicacid, 1,2,3,4-tetrahydro-, (S)-;(-)-1,2,3,4-Tetrahydroisoquinoline-3-carboxylicacid;(3S)-1,2,3,4-Tetrahydro-3-isoquinolinecarboxylic acid;(S)-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid;
- PSA 49.33000
- LogP 1.11430
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 90℃; for 12h; Pictet-Spengler Synthesis; | 98% |
| With hydrogenchloride In water at 90℃; for 12h; | 98% |
| With hydrogenchloride at 85℃; for 9h; Pictet-Spengler condensation; | 95% |
-
-
143767-56-0
(-)-menthyl (S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylate

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol for 6h; Ambient temperature; | 94% |
-
-
77497-96-2
(S)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid, phenylmethylester

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 15h; Ambient temperature; | 81% |
| With hydrogen; palladium on activated charcoal In ethanol; water; acetic acid for 4h; Ambient temperature; | 75.4% |
-
-
367952-43-0
(10aS)-3,3-Bis(trifluoromethyl)-1-oxo-(1,2,3,4-tetrahydroisoquinolino)[2,3-c]oxazolidine

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With water In isopropyl alcohol at 20℃; | 72% |
-
-
67123-97-1
(R,S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; borane-ammonia complex; recombinant Escherichia coli BL21 (DE3) cells expressed D-amino acid oxidase from Fusarium solani M-0718 In water at 30℃; pH=8; Resolution of racemate; Enzymatic reaction; enantioselective reaction; | 68% |
-
-
123053-49-6
(3R,10bS)-3-tert-Butyl-2-methyl-2,3,6,10b-tetrahydro-5H-imidazo[5,1-a]isoquinolin-1-one

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With hydrogenchloride; Dowex 50-WX8; acetic acid In water; toluene at 103℃; for 126h; | 67% |
-
-
307304-56-9
(3'S,4R,5S)-1,5-dimethyl-4-phenyl-3-(1',2',3',4'-tetrahydro-3'-isoquinolinylcarbonyl)-2-imidazolidinone

-
A
-
74163-81-8
L-Tic-OH

-
B
-
103733-65-9
(3R)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With water for 12h; Hydrolysis; epimerization; Heating; Title compound not separated from byproducts.; |
-
-
15912-55-7, 41234-43-9, 55857-63-1
ethyl 1,2,3,4-tetrahydroisoquinoline-3-(S)-carboxylate

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With chicken liver acetone; water pH=7.5; |
-
-
57060-86-3
1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With chicken liver acetone; water for 2h; pH=7.5; |

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With chicken liver acetone; water pH=7.5; |

| Conditions | Yield |
|---|---|
| Stage #1: α,α'-dibromo-o-xylene; chiral Ni complex of Schiff base of glycine and BPB With sodium hydroxide In acetonitrile at 20℃; for 0.666667h; Stage #2: In hydrogenchloride; methanol at 50℃; for 0.333333h; Stage #3: In ammonium hydroxide |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 91 percent / dimethylsulfoxide 2: 65 percent / SOCl2 3: BF3*OEt2 / 20 °C 4: 72 percent / H2O / propan-2-ol / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 91 percent / dimethylsulfoxide 2: 82 percent / TFA / CHCl3 / 0.5 h 3: 72 percent / H2O / propan-2-ol / 20 °C View Scheme |
-
-
150582-52-8
(4S)-4-Benzyl-2,2-bis(trifluoromethyl)-1,3-oxazolidin-5-one

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 65 percent / SOCl2 2: BF3*OEt2 / 20 °C 3: 72 percent / H2O / propan-2-ol / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 82 percent / TFA / CHCl3 / 0.5 h 2: 72 percent / H2O / propan-2-ol / 20 °C View Scheme |
-
-
367952-46-3
(4S)-4-Benzyl-2,2-bis(trifluoromethyl)-3-chloromethyl-1,3-oxazolidin-5-one

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: BF3*OEt2 / 20 °C 2: 72 percent / H2O / propan-2-ol / 20 °C View Scheme |
-
-
143767-54-8
(+/-)-2-acetyl-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 86 percent / 6 N aq. HCl / 4 h / Heating 2: 83 percent / p-TsOH*H2O / toluene / 30 h / Heating 3: 94 percent / NaOH / methanol / 6 h / Ambient temperature View Scheme |
-
-
612-12-4
o-Xylylene dichloride

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: NaOMe / 4 h / Heating 2: 6 N aq. HCl / 4 h / Heating 3: 83 percent / p-TsOH*H2O / toluene / 30 h / Heating 4: 94 percent / NaOH / methanol / 6 h / Ambient temperature View Scheme | |
| Multi-step reaction with 5 steps 1: NaOMe / 4 h / Heating 2: 1.) KOH, 2.) 2 N HCl / 1.) MeOH, H2O, reflux, 4 h, 2.) EtOAc, pH 1 3: 86 percent / 6 N aq. HCl / 4 h / Heating 4: 83 percent / p-TsOH*H2O / toluene / 30 h / Heating 5: 94 percent / NaOH / methanol / 6 h / Ambient temperature View Scheme |
-
-
143767-55-9
dimethyl 2-acetyl-1,2,3,4-tetrahydro-3,3-isoquinolinedicarboxylate

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 6 N aq. HCl / 4 h / Heating 2: 83 percent / p-TsOH*H2O / toluene / 30 h / Heating 3: 94 percent / NaOH / methanol / 6 h / Ambient temperature View Scheme | |
| Multi-step reaction with 4 steps 1: 1.) KOH, 2.) 2 N HCl / 1.) MeOH, H2O, reflux, 4 h, 2.) EtOAc, pH 1 2: 86 percent / 6 N aq. HCl / 4 h / Heating 3: 83 percent / p-TsOH*H2O / toluene / 30 h / Heating 4: 94 percent / NaOH / methanol / 6 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 75 percent / NaOMe / 4 h / Heating 2: 6 N aq. HCl / 4 h / Heating 3: 83 percent / p-TsOH*H2O / toluene / 30 h / Heating 4: 94 percent / NaOH / methanol / 6 h / Ambient temperature View Scheme | |
| Multi-step reaction with 5 steps 1: 75 percent / NaOMe / 4 h / Heating 2: 1.) KOH, 2.) 2 N HCl / 1.) MeOH, H2O, reflux, 4 h, 2.) EtOAc, pH 1 3: 86 percent / 6 N aq. HCl / 4 h / Heating 4: 83 percent / p-TsOH*H2O / toluene / 30 h / Heating 5: 94 percent / NaOH / methanol / 6 h / Ambient temperature View Scheme |
-
-
150-30-1
Phenylalanine

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 69 percent / conc. HCl View Scheme |

| Conditions | Yield |
|---|---|
| In hydrogenchloride; ethanol; water |

-
-
50-00-0
formaldehyd

-
-
1009-67-2, 5628-72-8, 14367-54-5, 14367-67-0
2-methyl-3-phenylpropionic acid

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water Reflux; |
-
-
67-56-1
methanol

-
-
74163-81-8
L-Tic-OH

-
-
79815-19-3
methyl (3S)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylate

| Conditions | Yield |
|---|---|
| With thionyl chloride | 100% |
| With thionyl chloride at 0 - 20℃; | 96% |
| With sulfuric acid for 12h; Reflux; | 95% |
-
-
541-41-3
chloroformic acid ethyl ester

-
-
74163-81-8
L-Tic-OH

-
-
104882-53-3
2-carboethoxy-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In tetrahydrofuran; water 1.) 0 deg C to room temperature, 2.) room temperature, 3 h; | 100% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
74163-81-8
L-Tic-OH

-
-
115962-35-1, 144069-68-1, 78879-20-6
(3S)-2-tert-butoxycarbonyl-1,2,3,4-tetrahydroisoquinole-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane; water at 20℃; for 144h; | 100% |
| With sodium hydrogencarbonate In 1,4-dioxane; water at 20℃; for 144h; Time; | 100% |
| With sodium hydrogencarbonate In 1,4-dioxane; water at 20℃; for 144h; Concentration; | 100% |
-
-
67-56-1
methanol

-
-
74163-81-8
L-Tic-OH

-
-
78183-55-8
(S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, methyl ester, hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride | 100% |
| With thionyl chloride at 0 - 40℃; for 21h; Inert atmosphere; | 99% |
| With thionyl chloride at 0 - 40℃; for 21h; Inert atmosphere; | 99% |
| With chloro-trimethyl-silane at 70℃; for 2h; Reflux; |
-
-
74163-81-8
L-Tic-OH

-
-
501-53-1
benzyl chloroformate

-
-
79261-58-8
(S)-3,4-dihydro-1H-isoquinoline-2,3-dicarboxylic acid 2-benzyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water at 20℃; for 16h; | 99% |
| With sodium hydroxide In 1,4-dioxane; water at 0 - 20℃; for 16.5h; | 99% |
| With sodium hydrogencarbonate In water at 20℃; for 1.5h; | 97% |
-
-
74163-81-8
L-Tic-OH

-
-
18881-17-9, 62855-02-1, 62928-94-3, 63006-93-9
(S)-3-hydroxymethyl-1,2,3,4-tetrahydroisoquinoline

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex; boron trifluoride diethyl etherate In tetrahydrofuran for 6h; Reduction; Heating; | 96% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 6h; Heating; | 86% |
| With dimethylsulfide borane complex; boron trifluoride diethyl etherate In tetrahydrofuran | 78% |
-
-
75-44-5
phosgene

-
-
74163-81-8
L-Tic-OH

-
-
186606-17-7
(10aS)-10,10a-dihydro[1,3]oxazolo[3,4-b]isoquinoline-1,3,(5H)-dione

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; dichloromethane at -30 - 20℃; | 95% |
-
-
74163-81-8
L-Tic-OH

-
-
77141-07-2
(S)-7-nitro-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at -20℃; for 0.5h; | 93% |
| With sulfuric acid; nitric acid at -10℃; for 3.5h; | 90% |
| With sulfuric acid; potassium nitrate at 5 - 20℃; |
-
-
50-00-0
formaldehyd

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With formic acid for 4h; Reflux; | 93% |
-
-
50-00-0
formaldehyd

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; L-Tic-OH With formic acid In water for 4h; Reflux; Stage #2: With hydrogenchloride In water Cooling with ice; | 93% |
-
-
64-17-5
ethanol

-
-
74163-81-8
L-Tic-OH

-
-
15912-55-7
ethyl (S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylate

| Conditions | Yield |
|---|---|
| With thionyl chloride Reflux; | 91% |
| With thionyl chloride | |
| With sulfuric acid at 80℃; for 20h; Inert atmosphere; |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
100-52-7
benzaldehyde

-
-
74163-81-8
L-Tic-OH

-
-
1346425-09-9
2-benzyl-1,2,3,4-tetrahydroisoquinoline-3-carbonitrile

| Conditions | Yield |
|---|---|
| In butan-1-ol at 200℃; for 0.166667h; decarboxylative Strecker reaction; Microwave irradiation; enantioselective reaction; | 91% |

-
-
35661-60-0
Fmoc-Leu-OH

-
-
71989-31-6
Fmoc-Pro-OH

-
-
35661-40-6
N-Fmoc L-Phe

-
-
71989-16-7
Fmoc-Asn-OH

-
-
91000-69-0
Fmoc-L-Arg-OH

-
-
74163-81-8
L-Tic-OH

-
-
76-05-1
trifluoroacetic acid

| Conditions | Yield |
|---|---|
| Stage #1: Fmoc-Asn-OH With benzotriazol-1-ol; diisopropyl-carbodiimide In N,N-dimethyl-formamide for 2h; Stage #2: With piperidine In N,N-dimethyl-formamide for 0.333333h; Stage #3: Fmoc-Leu-OH; Fmoc-Pro-OH; N-Fmoc L-Phe; Fmoc-L-Arg-OH; L-Tic-OH; trifluoroacetic acid Further stages; | 89% |

-
-
12354-84-6, 12354-85-7
bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With CH3ONa In methanol; dichloromethane (Ar); addn. of a soln. of MeONa to a soln. of ligand in methanol, addn. to a soln. of iridium complex in CH2Cl2, stirring for 3 h; evapn., dissolving in CH2Cl2, filtration, concn., addn. of Et2O, filtration, washing with Et2O, pentane, drying in vac.; elem. anal.; | 85% |
-
-
6046-93-1
copper(II) acetate monohydrate

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| In methanol (Ar); addn. of ligand to a soln. of copper salt in methanol; isolation, washing with methanol, ether, drying in vac.; elem. anal.; | 84% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 120℃; for 16h; Inert atmosphere; | 84% |
-
-
74163-81-8
L-Tic-OH

-
-
877932-98-4
N-(1-ethoxycarbonyl-3-phenylpropyl)-L-alanine

-
-
129178-93-4
L-alanine acid chloride

-
-
85441-61-8
quinapril

| Conditions | Yield |
|---|---|
| With benzenesulfonamide; phosphorus pentachloride; triethylamine In n-heptane; dichloromethane; water | 83.9% |

-
-
74163-81-8
L-Tic-OH

-
-
6624-49-3
isoquinoline-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate In N,N-dimethyl-formamide at 0 - 20℃; for 100.5h; Inert atmosphere; | 83% |
| With potassium permanganate | 83% |
| With potassium permanganate In N,N-dimethyl-formamide at 20℃; for 72h; Cooling with ice; | 62% |

| Conditions | Yield |
|---|---|
| In methanol (Ar); addn. of ligand to a soln. of copper salt in methanol; isolation, washing with methanol, ether, drying in vac.; elem. anal.; | 81% |
-
-
1070-19-5
N-(tert-butyloxycarbonyl) azide

-
-
74163-81-8
L-Tic-OH

-
-
115962-35-1, 144069-68-1, 78879-20-6
(3S)-2-tert-butoxycarbonyl-1,2,3,4-tetrahydroisoquinole-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 48h; pH=10; | 80% |
-
-
112-77-6
(Z)-9-octadecenoyl chloride

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; acetone at 0 - 20℃; | 78% |
-
-
23147-58-2, 110822-84-9, 110822-85-0
glycolaldehyde dimer

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| In 2,2,2-trifluoroethanol at -40 - 20℃; for 12h; Ugi reaction; | 77% |

-
-
52462-29-0
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With CH3ONa In methanol; dichloromethane (Ar); addn. of a soln. of MeONa to a soln. of ligand in methanol, addn. to a soln. of ruthenium complex in CH2Cl2, stirring for 3 h; evapn., dissolving in CH2Cl2, filtration through celite, concn., addn. to pentane, filtration, washing with pentane, drying in vac.; elem. anal.; | 77% |
-
-
15692-96-3
trans-di-μ-chloro-dichlorobis(triethylphosphine)diplatinum

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With CH3ONa In methanol; dichloromethane (Ar); addn. of a soln. of MeONa to a soln. of ligand in methanol, addn. to a soln. of platinum complex in CH2Cl2, stirring for 3 h; evapn., dissolving in CH2Cl2, filtration, addn. of pentane, washing withpentane, drying in vac.; elem. anal.; | 77% |

-
-
74163-81-8
L-Tic-OH

| Conditions | Yield |
|---|---|
| With CH3ONa In methanol; dichloromethane (Ar); addn. of a soln. of MeONa to a soln. of ligand in methanol, addn. to a soln. of palladium complex in CH2Cl2, stirring for 3 h; evapn., dissolving in CH2Cl2, filtration, evapn., addn. of pentane, cooling to -20°C, washing with pentane, drying in vac.; elem. anal.; | 76% |
-
-
13139-17-8
N-(Benzyloxycarbonyloxy)succinimide

-
-
74163-81-8
L-Tic-OH

-
-
79261-58-8
(S)-3,4-dihydro-1H-isoquinoline-2,3-dicarboxylic acid 2-benzyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl-formamide at 0 - 20℃; for 27h; | 74.7% |
L-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid Chemical Properties
IUPAC Name: (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
Synonyms of L-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid (CAS NO.74163-81-8): 3-Isoquinolinecarboxylicacid, 1,2,3,4-tetrahydro-, (S)- ; (3S)-1,2,3,4-Tetrahydro-3-isoquinolinecarboxylic acid ; S-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid
CAS NO: 74163-81-8
Molecular Formula: C10H11NO2
Molecular Weight: 177.20
Molecular Structure: 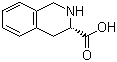
H bond acceptors: 3
H bond donors: 2
Freely Rotating Bonds: 1
Polar Surface Area: 29.54 Å2
Index of Refraction: 1.576
Molar Refractivity: 47.88 cm3
Molar Volume: 144.5 cm3
Surface Tension: 48.2 dyne/cm
Density: 1.225 g/cm3
Flash Point: 178.8 °C
Enthalpy of Vaporization: 65.31 kJ/mol
Boiling Point: 372 °C at 760 mmHg
Vapour Pressure: 3.41E-06 mmHg at 25°C
Melting point: 300 °C
Alpha: -157.4 º (24°C)
Storage temp: Store at 0-5°C
Appearance: white to light yellow crystal powder
Product Categories of L-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid (CAS NO.74163-81-8): Amino Acids;Carboxylic Acids;Quinolines, Isoquinolines & Quinoxalines;chiral;(intermediate of quinapril);Carboxylic Acids;Quinolines, Isoquinolines & Quinoxalines;IsoquinolinesPeptide Synthesis;Chiral Building Blocks;Heterocyclic Building Blocks;Others;Unnatural Amino Acid Derivatives;Peptide Synthesis;a-amino
L-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid Safety Profile
Safety Information about L-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid (CAS NO.74163-81-8):
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36/37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany: 3
L-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid Specification
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or chemical foam
Handling: Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage: Store in a cool, dry place. Store in a tightly closed container.
Related Products
- L-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid
- L-1,2,3,4-Tetrahydronorharman-3-carboxylic acid
- L-1,2,3,4-Tetrahydro-quinoline-2-carboxylic acid hydrochloride
- L-1-Boc-Nipecotic acid
- L-1-Cbz-pipecolinamide
- L-1-Naphthylalanine
- 74163-84-1
- 74164-84-4
- 74165-64-3
- 7416-57-1
- 741667-07-2
- 741681-54-9
- 741686-49-7
- 741687-07-0
- 741698-80-6
- 741709-56-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View