-
Name
L-Carnosine
- EINECS 206-169-9
- CAS No. 305-84-0
- Article Data29
- CAS DataBase
- Density 1.41 g/cm3
- Solubility almost transparency
- Melting Point 253 °C (dec.)(lit.)
- Formula C9H14N4O3
- Boiling Point 511.7 °C at 760 mmHg
- Molecular Weight 226.235
- Flash Point 263.3 °C
- Transport Information
- Appearance crystalline
- Safety 24/25-36-26
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms beta-Alanyl-L-histidine;L-Carnosine 99% min.;N-2-M;L-Carnosinc;β-Alanyl-L-histidine;L-Carnosine Acetate;Karnozin;2-(3-aminopropanoylamino)-3-(3H-imidazol-4-yl)propanoic acid;L-Histidine, N-.beta.-alanyl-;Nalpha-(beta-alanyl)-L-histidine;(2S)-2-(3-aminopropanoylamino)-3-(3H-imidazol-4-yl)propanoic acid;.beta.-Alanyl-L-histidine;Ignotine;
- PSA 121.10000
- LogP -0.03840
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: N-9-fluorenylmethyloxycarbonyl-N-trityl-L-histidine With trityl chloride polystyrene resin; N-ethyl-N,N-diisopropylamine In dichloromethane; N,N-dimethyl-formamide for 0.5h; trityl chloride resin; Stage #2: With piperazine In dichloromethane; N,N-dimethyl-formamide for 1h; trityl chloride resin; Inert atmosphere; Stage #3: 3-(tert-butyloxycarbonylamino)propionic acid Further stages; | 90% |

| Conditions | Yield |
|---|---|
| With recombinant β-aminopeptidase BapA from Sphingosinicella xenopeptidilytica 3-2W4 at 37℃; pH=10; Kinetics; Reagent/catalyst; pH-value; aq. buffer; Enzymatic reaction; | 53% |
-
-
64017-81-8
3-aminopropanamide hydrochloride

-
-
7389-87-9
L-histidine methyl ester dihydrochloride

-
A
-
2140-53-6
3-(3-Amino-propionylamino)-propionic acid

-
B
-
1246364-63-5
H-(β-Ala)3-NH2

-
C
-
1246364-64-6
H-β-Ala-β-Ala-L-His-OH

-
E
-
305-84-0
carnosine

| Conditions | Yield |
|---|---|
| With recombinant β-aminopeptidase DmpA from Ochrobactrum anthropi LMG7991 at 37℃; for 2h; pH=10; aq. buffer; Enzymatic reaction; |


| Conditions | Yield |
|---|---|
| With recombinant β-aminopeptidase BapA from Sphingosinicella xenopeptidilytica 3-2W4 at 37℃; pH=10; Kinetics; Reagent/catalyst; pH-value; aq. buffer; Enzymatic reaction; |

-
-
305-84-0
carnosine

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 95℃; for 0.116667h; pH=12; aq. buffer; |

| Conditions | Yield |
|---|---|
| With 1-hydroxy-pyrrolidine-2,5-dione; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In water; acetonitrile at 4℃; for 0.2h; pH=7.5; Reagent/catalyst; Solvent; pH-value; |

| Conditions | Yield |
|---|---|
| Stage #1: 4-(((L-histidyl)oxy)methyl)-N,N,N-trimethylbenzenaminium; Smoc-β-alanine With 1-hydroxy-pyrrolidine-2,5-dione; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In water; isopropyl alcohol for 0.25h; pH=7.5; Stage #2: With piperazine In water; isopropyl alcohol |

| Conditions | Yield |
|---|---|
| With thionyl chloride for 1h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In methanol at 25 - 60℃; for 3h; Solvent; | 99.7% |
| With sodium hydroxide In water at 23 - 83℃; for 3h; Solvent; Temperature; | 96.8% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
305-84-0
carnosine

-
-
405520-71-0
(S)-3-(1-(tert-butoxycarbonyl)-1H-imidazol-5-yl)-2-(3-((tert-butoxycarbonyl)amino)propanamido)propanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: di-tert-butyl dicarbonate; carnosine With sodium hydroxide In 1,3-dioxane; water at 0 - 20℃; Inert atmosphere; Stage #2: With potassium dihydrogenphosphate In 1,3-dioxane; water | 76% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; iodine In ethanol for 1.5h; | A 72% B n/a |

| Conditions | Yield |
|---|---|
| Stage #1: carnosine With sodium hydrogencarbonate In water; N,N-dimethyl-formamide for 0.5h; Stage #2: C59H90N4O7 In water; N,N-dimethyl-formamide | 68% |
-
-
305-84-0
carnosine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate at 60℃; for 12h; | 30% |
-
-
305-84-0
carnosine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; N-Bromosuccinimide; tiolacetic acid In water at 0℃; for 1h; | 14% |
-
-
305-84-0
carnosine

-
-
125347-29-7
(S)-2-(3-Hydroxy-propionylamino)-3-(3H-imidazol-4-yl)-propionic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite In water for 24h; Ambient temperature; | 100 % Spectr. |
-
-
51908-29-3
4-isothiocyanatobenzene sulfonamide

-
-
305-84-0
carnosine

| Conditions | Yield |
|---|---|
| In acetone Heating; |

| Conditions | Yield |
|---|---|
| In phosphate buffer at 37℃; pH=7.4; Product distribution; Michael addition; |


| Conditions | Yield |
|---|---|
| In phosphate buffer at 37℃; pH=7.4; Product distribution; Further Variations:; Reaction partners; time dependence; |


-
-
305-84-0
carnosine

| Conditions | Yield |
|---|---|
| With potassium nitrate In water reaction of copper nitrate and l-carnosine in water at 25°C in presence of KNO3 at different pH (3-8) and metal-ligand ratios;; not isolated; detn. by UV;; |

-
-
1284258-81-6
1-{4-[(4-aminobutylaminooxy)methyl]-2,3-dichlorophenyl}-2-methylenebutan-1-one

-
-
305-84-0
carnosine

-
-
1284260-28-1
C26H34Cl2N6O5

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide Inert atmosphere; |
-
-
305-84-0
carnosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydroxide / 1,3-dioxane; water / 0 - 20 °C / Inert atmosphere 2: triethylamine; 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide / tetrahydrofuran; ethyl acetate / 20 °C / Inert atmosphere 3: hydrogenchloride / 1,3-dioxane / 20 °C / Inert atmosphere View Scheme |
-
-
305-84-0
carnosine

-
-
1538576-75-8
tert-butyl 5-((S)-3-((R)-2-acetamido-3-amino-3-oxopropylthio)-2-(3-(tert-butoxycarbonylamino)propanamido)-3-oxopropyl)-1H-imidazole-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide / 1,3-dioxane; water / 0 - 20 °C / Inert atmosphere 2: triethylamine; 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide / tetrahydrofuran; ethyl acetate / 20 °C / Inert atmosphere View Scheme |
-
-
305-84-0
carnosine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydroxide / 1,3-dioxane; water / 0 - 20 °C / Inert atmosphere 2: benzotriazol-1-ol; dicyclohexyl-carbodiimide / ethyl acetate / 0 - 20 °C / Inert atmosphere 3: hydrogenchloride / 1,3-dioxane / 20 °C View Scheme |
-
-
305-84-0
carnosine

-
-
1538576-76-9
5-[2-(2-acetylamino-2-methoxycarbonylethylsulfanylcarbonyl)-2-(3-tert-butoxycarbonylamino-propionylamino)ethyl]imidazole-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide / 1,3-dioxane; water / 0 - 20 °C / Inert atmosphere 2: benzotriazol-1-ol; dicyclohexyl-carbodiimide / ethyl acetate / 0 - 20 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| In aq. phosphate buffer at 37℃; pH=7.4; |

| Conditions | Yield |
|---|---|
| In aq. phosphate buffer at 37℃; pH=7.4; |


| Conditions | Yield |
|---|---|
| In aq. phosphate buffer at 37℃; pH=7.4; |
-
-
850480-50-1
4-hydroxynon-2-enal

-
-
305-84-0
carnosine

| Conditions | Yield |
|---|---|
| In aq. phosphate buffer at 37℃; pH=7.4; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 50 - 60℃; for 1h; Temperature; | 12.8 g |
-
-
305-84-0
carnosine

| Conditions | Yield |
|---|---|
| With copper(II) sulfate; ascorbic acid; oxygen-18 In aq. phosphate buffer at 20℃; for 0.0833333h; pH=7.2; Solvent; |
-
-
305-84-0
carnosine

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) sulfate; ascorbic acid In aq. phosphate buffer at 20℃; for 0.5h; pH=7.2; Solvent; |
L-Carnosine Chemical Properties
Product Name: L-Carnosine
Synonyms: l-Ignotine ; l-Beta-alanine histidine ; Beta-alanyl-l-histidine ; Beta-ala-l-his ; L-Carnosine ; b-Alanyl-l-histidine ; Beta-a-h ; h-Beta-ala-his-oh
IUPAC Name: 2-(3-aminopropanoylamino)-3-(1H-imidazol-5-yl)propanoic acid
The Molecular formula of Carnosine (136572-09-3): C9H14N4O3
The Molecular Weight of Carnosine (136572-09-3): 226.23 g/mol
Molecular Structure : 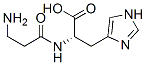
EINECS: 206-169-9
Melting point: 253 °C (dec.)(lit.)
alpha: 20.9 o (c=1.5, H2O)
storage temp.: 2-8°C
Appearance: Crystalline
Density: 1.41 g/cm3
Flash Point: 263.3 °C
Index of Refraction: 1.607
Surface Tension: 76.9 dyne/cm
Enthalpy of Vaporization: 82.42 kJ/mol
Boiling Point: 511.7 °C at 760 mmHg
Vapour Pressure: 2.69E-11 mmHg at 25 °C
L-Carnosine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 9087mg/kg (9087mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | United States Patent Document. Vol. #4446149, |
| mouse | LD50 | oral | > 14930mg/kg (14930mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | United States Patent Document. Vol. #4446149, |
CAS# 305-84-0: Oral, mouse: LD50 = >14930 mg/kg;
Carcinogenicity: L-Carnosine - Not listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65.
Other: The toxicological properties have not been fully investigated. See actual entry in RTECS for complete information.
L-Carnosine Consensus Reports
Reported in EPA TSCA Inventory.
L-Carnosine Safety Profile
Mildly toxic by intraperitoneal route. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx.
The Hazard Codes of CARNOSINE(305-84-0):  Xn
Xn
The Risk Statements information of Carnosine (305-84-0): 20/21/22-36/37/38
20/21/22: Harmful by inhalation, in contact with skin and if swallowed
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements information of Carnosine (305-84-0): 24/25-36-26
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
24/25: Avoid contact with skin and eyes
WGK Germany: 2
RTECS: MS3080000
F: 3-10
L-Carnosine Specification
Chemical Stability: Stable under normal temperatures and pressures.
Conditions to Avoid: Incompatible materials.
Incompatibilities with Other Materials Strong oxidizing agents.
Hazardous Decomposition Products Carbon monoxide, carbon dioxide, nitrogen oxides (NOx) and ammonia (NH3).
Hazardous Polymerization Will not occur.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View