-
Name
Lidocaine hydrochloride
- EINECS 200-803-8
- CAS No. 73-78-9
- Article Data4
- CAS DataBase
- Density
- Solubility
- Melting Point 80-82 °C
- Formula C14H22N2O.HCl
- Boiling Point 350.8 °C at 760 mmHg
- Molecular Weight 270.802
- Flash Point 166 °C
- Transport Information
- Appearance white crystal powder
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2',6'-Acetoxylidide,2-(diethylamino)-, monohydrochloride (8CI);Acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-,monohydrochloride (9CI);2-(Diethylamino)-2',6'-dimethylacetanilide hydrochloride;Alphacaine;DioCaine;Irtopan;Lidesthesin;Lidocain hydrochloride;Lidocaine monohydrochloride;Lidothesin;Lignavet;Luan;Metaclopromide hydrochloride;Odontalg;Proliferol;Sedagul;Versicane;Xylocaine Astra;Xylocard;Xylotox;Lidocaine HCl .1H2O;
- PSA 32.34000
- LogP 3.45870
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether | 100% |
| With hydrogenchloride; pyrographite In water; 1,2-dichloro-ethane for 0.333333h; pH=3.5; pH-value; | 88.72% |
| With hydrogenchloride In water; acetone at 20℃; pH=<= 4; | 80.6% |
-
-
109-89-7
diethylamine

-
-
79-04-9
chloroacetyl chloride

-
-
87-62-7
2,6-dimethylaniline

-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: chloroacetyl chloride; 2,6-dimethylaniline In 1-methyl-pyrrolidin-2-one; methanol; water at 120℃; for 0.306667h; Flow reactor; Stage #2: diethylamine With potassium hydroxide In methanol; water at 130℃; under 12751.3 Torr; for 0.295h; Flow reactor; Stage #3: With hydrogenchloride In methanol; diethyl ether; water |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ammonium hydroxide; 2,6-dimethylcyclohexanone; 5%-palladium/activated carbon / 5 h / 180 °C 2: sodium methylate / 0.5 h / 95 °C 3: hydrogenchloride; pyrographite / 1,2-dichloro-ethane; water / 0.33 h / pH 3.5 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / acetone / 3 h / 20 °C 2: acetone / 8 h / Reflux 3: hydrogenchloride / water; acetone / 20 °C / pH <= 4 View Scheme |

| Conditions | Yield |
|---|---|
| In acetonitrile at 50℃; for 4h; Sonication; | 99% |
-
-
55589-62-3
potassium acesulfame

-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| In acetonitrile at 50℃; for 4h; Sonication; | 98% |

| Conditions | Yield |
|---|---|
| In methanol; water at 50℃; for 4h; | 97% |

| Conditions | Yield |
|---|---|
| In methanol; water at 50℃; for 4h; | 96% |
-
-
73-78-9
lidocaine hydrochloride

-
-
32845-42-4
1-ethyl-2-methyl-3-(2,6-dimethylphenyl)imidazolidin-4-one

| Conditions | Yield |
|---|---|
| With glucose dehydrogenase; D-glucose; cytochrome P450BM3 R47L/Y51F/A191T/N239H/I259V/A276T/A330W/L353I mutant; oxygen; β-nicotinamide adenine dinucleotide phosphate sodium salt In ethanol at 25℃; for 2h; pH=8.5; Enzymatic reaction; | 95% |
| Multi-step reaction with 2 steps 1: 55 percent / H2O2, imidazole / chloromanganese(III) / CH2Cl2; acetonitrile 2: 80 percent / CH2Cl2; acetonitrile / 3 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| In dichloromethane; water for 2h; Sonication; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water at -4 - 0℃; under 760.051 Torr; | 93% |

| Conditions | Yield |
|---|---|
| In water; acetone at 20℃; | 87% |
| In water; acetone at 20℃; | 87% |
| In water; acetone at 20℃; Product distribution / selectivity; | 54% |

| Conditions | Yield |
|---|---|
| With glucose dehydrogenase; D-glucose; cytochrome P450BM3 R47L/Y51F/F81W/F87V/E267V/I401P mutant; β-nicotinamide adenine dinucleotide phosphate sodium salt In ethanol at 25℃; for 2h; pH=8.5; Enzymatic reaction; | 85% |
| With 1H-imidazole; dihydrogen peroxide; chloro(meso-tetrakis(2,6-dichlorophenyl)porphyrinato)manganese(III) In dichloromethane; acetonitrile | 55% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; acetone at 0℃; | 83% |
-
-
73-78-9
lidocaine hydrochloride

-
-
31121-93-4
sodium 2-(4-isobutylphenyl)propionate

-
-
1158170-79-6
lidocainium ibuprofenate

| Conditions | Yield |
|---|---|
| In water at 20℃; for 4h; | 75.7% |
-
-
333-20-0
potassium thioacyanate

-
-
6018-89-9
nickel(II) acetate tetrahydrate

-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; water at 20℃; for 4h; pH=7; | 72.5% |

-
-
6018-89-9
nickel(II) acetate tetrahydrate

-
-
1934-75-4
sodium dicyanamide

-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; water at 20℃; for 4h; pH=7; | 68% |

-
-
333-20-0
potassium thioacyanate

-
-
6147-53-1
cobalt(II) diacetate tetrahydrate

-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; water at 20℃; for 4h; pH=7; | 65.6% |

-
-
6147-53-1
cobalt(II) diacetate tetrahydrate

-
-
1934-75-4
sodium dicyanamide

-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; water at 20℃; for 4h; pH=7; | 62% |

-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 55 percent / H2O2, imidazole / chloromanganese(III) / CH2Cl2; acetonitrile 2: 80 percent / iodosylbenzene, imidazole / chloroiron(III) / CH2Cl2; acetonitrile / 3 h / 20 °C View Scheme |
-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 55 percent / H2O2, imidazole / chloromanganese(III) / CH2Cl2; acetonitrile 2: 1.) iodosylbenzene, imidazole / 1.) chloroiron(III) / 1.) CH2Cl2, CH3CN, 20 deg C, 3 h, 2.) RT, 3 h View Scheme |
-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 55 percent / H2O2, imidazole / chloromanganese(III) / CH2Cl2; acetonitrile 2: 86 percent / CH2Cl2 / 2 h / 20 °C View Scheme |
-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 55 percent / H2O2, imidazole / chloromanganese(III) / CH2Cl2; acetonitrile 2: 18 percent / iodosylbenzene, imidazole / chloroiron(III) / CH2Cl2; acetonitrile / 3 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| In methanol for 2h; | |
| In ethanol; dichloromethane at 50℃; Inert atmosphere; |

-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| In ethanol ligand added to soln. of Pt-compound; heated at 60-80°C for ca. 30 min;; filtration; washing (EtOH then ether); drying (vacuum); recrystn. (EtOH) by slow cooling and evapn.; |

| Conditions | Yield |
|---|---|
| at 80℃; |
-
-
73-78-9
lidocaine hydrochloride

-
A
-
7728-40-7
N-(2,6-dimethylphenyl)-2-ethylaminoacetamide

-
B
-
32845-42-4
1-ethyl-2-methyl-3-(2,6-dimethylphenyl)imidazolidin-4-one

| Conditions | Yield |
|---|---|
| With glucose dehydrogenase; D-glucose; cytochrome P450BM3 R47L/Y51F/F87V/E267V/I401P mutant; β-nicotinamide adenine dinucleotide phosphate sodium salt In ethanol at 25℃; for 2h; pH=8.5; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| In aq. phosphate buffer pH=7.4; UV-irradiation; |
-
-
73-78-9
lidocaine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogen (18)O-peroxide In water for 0.0166667h; |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water for 0.0166667h; |
Lidocaine hydrochloride Chemical Properties
IUPAC Name: 2-(Diethylamino)-N-(2,6-dimethylphenyl)acetamide hydrochloride
Molecular Formula: C14H23ClN2O
Molecular Weight: 270.7982
EINECS: 200-803-8
Melting Point: 80-82°C
Boiling Point: 350.8 °C at 760 mmHg
Flash Point: 166 °C
Freely Rotating Bonds: 5
Polar Surface Area: 23.55 Å2
Enthalpy of Vaporization: 59.55 kJ/mol
Vapour Pressure: 4.28E-05 mmHg at 25°C
Appearance: white crystal powder
The Cas Register Number of 2-Diethylamino-2',6'-acetoxylidide hydrochloride is 73-78-9. The chemical synonyms of 2-Diethylamino-2',6'-acetoxylidide hydrochloride (CAS NO.73-78-9) are 2-Diethylamino-n-(2'6'-dimethylphenyl)acetamide HCl ; 2-Diethylamino-n-[2,6-dimethylphenyl]acetamide HCl ; alpha-(Diethylamino)-2',6'-acetoxylidide hydrochloride ; a-(Diethylamino)-2 6-dimethylacetanilide hydrochloride ; Lignocaine hydrochloride ; Lidocaine hydrochloride ; Lidocaine HCl and 2-(diethylamino)-n-(2,6-dimethylphenyl)-acetamidmonohydrochloride . The product Categories are API , Intermediates , Fine Chemicals and Pharmaceuticals . The molecular structure of 2-Diethylamino-2',6'-acetoxylidide hydrochloride (CAS NO.73-78-9) is
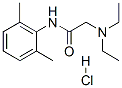 .
.
Lidocaine hydrochloride Uses
2-Diethylamino-2',6'-acetoxylidide hydrochloride (CAS NO.73-78-9) can be used as a local anesthetics, antiarrhythmic (class IB) drugs, a variety of anesthesia and rapid ventricular arrhythmia.Long-acting, membrane stabilizing agent against ventricular arrhythmia.
Lidocaine hydrochloride Toxicity Data With Reference
| 1. | skn-rbt 3% MLD | AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. 137 (1962),410. | ||
| 2. | eye-rbt 3% MLD | AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. 137 (1962),410. | ||
| 3. | ivn-cld TDLo:60 mg/kg/1H | JTCTDW Journal of Toxicology, Clinical Toxicology. 24 (1986),51. | ||
| 4. | ivn-man TDLo:9 mg/kg/4H-C:CVS | DICPBB Drug Intelligence and Clinical Pharmacy. 19 (1985),669. | ||
| 5. | ivn-man TDLo:7143 µg/kg:BPR | CHETBF Chest: The Journal of Circulation, Respiration and Related Systems. 61 (1972),682. | ||
| 6. | imp-man TDLo:5714 µg/kg | CMAJAX Canadian Medical Association Journal. 137 (1987),219. | ||
| 7. | ipr-rat LD50:122 mg/kg | JPETAB Journal of Pharmacology and Experimental Therapeutics. 111 (1954),224. | ||
| 8. | scu-rat LD50:570 mg/kg | RPOBAR Research Progress in Organic Biological and Medicinal Chemistry. 2 (1970),299. | ||
| 9. | ivn-rat LD50:21 mg/kg | RPOBAR Research Progress in Organic Biological and Medicinal Chemistry. 2 (1970),299. | ||
| 10. | orl-mus LD50:220 mg/kg | RPOBAR &nbs |
Lidocaine hydrochloride Consensus Reports
Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
Lidocaine hydrochloride Safety Profile
Poison by ingestion, intraperitoneal, intravenous, subcutaneous, intramuscular, and intratracheal routes. Human systemic effects: somnolence, respiratory depression, low blood pressure, cardiomyopathy including infarction, pulse rate increase. An experimental teratogen. Other experimental reproductive effects. A skin and eye irritant. An anesthetic. When heated to decomposition it emits very toic fumes of NOx and HCl. Its hazard class is 6.1(b).
RIDADR: 3249
HazardClass: 6.1(b)
PackingGroup: III
Related Products
- Lidocaine
- Lidocaine hydrochloride
- 7378-99-6
- 73790-05-3
- 73790-06-4
- 73790-28-0
- 73790-48-4
- 7379-12-6
- 73791-47-6
- 73791-65-8
- 73792-22-0
- 7379-35-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View