-
Name
Mangostin
- EINECS 448-420-7
- CAS No. 6147-11-1
- Article Data5
- CAS DataBase
- Density 1.265 g/cm3
- Solubility
- Melting Point 180-182 °C
- Formula C24H26O6
- Boiling Point 640.1 °C at 760 mmHg
- Molecular Weight 410.467
- Flash Point 220.3 °C
- Transport Information
- Appearance Yellow crystalline solid
- Safety 45
- Risk Codes 25
-
Molecular Structure
- Hazard Symbols T
- Synonyms 9H-Xanthen-9-one,1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)- (9CI);Mangostin(6CI,7CI);Xanthen-9-one,1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)- (8CI);1,3,6-Trihydroxy-7-methoxy-2,8-bis(3,3-dimethylallyl)xanthone;NSC 139154;a-Mangosten;a-Mangostin;
- PSA 100.13000
- LogP 5.08900
Synthetic route

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 1h; Concentration; Reagent/catalyst; Solvent; Reflux; | 57.1% |
| With potassium hydroxide |
-
-
15404-76-9
dimethylmangostin

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| With 1-thiopropane; sodium hydride In N,N-dimethyl-formamide for 6h; Inert atmosphere; Reflux; | 60% |
| With sodium methylate In dimethyl sulfoxide for 6h; Reflux; Inert atmosphere; | 59.8% |
-
-
107389-90-2
1,3,6-tri-O-acetyl-α-mangostin

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 1h; Concentration; Reagent/catalyst; Solvent; Reflux; | 57.1% |
| With potassium hydroxide |
-
-
31271-10-0
6,8-dihydroxy-2-methoxy-1,7-bis(3-methylbut-2-en-1-yl)-9-oxo-9H-xanthen-3-yl acetate

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 1h; Reflux; | 50.4% |
-
-
1140525-42-3
1,3-dihydroxy-6-O-benzoyl-7-methoxy-2,8-bis(3-methyl-2-butenyl)-9-xanthone

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 1h; Reflux; | 44% |
-
-
426820-49-7
[4,6-dihydroxy-2-methoxymethoxy-3-(3-methyl-but-2-enyl)-phenyl]-[4,6-dihydroxy-3-methoxy-2-(3-methyl-but-2-enyl)-phenyl]-methanone

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Stage #1: [4,6-dihydroxy-2-methoxymethoxy-3-(3-methyl-but-2-enyl)-phenyl]-[4,6-dihydroxy-3-methoxy-2-(3-methyl-but-2-enyl)-phenyl]-methanone With tetrachloromethane; triphenylphosphine In tetrahydrofuran at 20℃; Stage #2: With silica gel | 43% |
-
-
1449384-89-7
3,6-di-O-benzoyl-α-mangostin

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 1h; Reflux; | 40% |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1.1: 96 percent / NaH / dimethylformamide / 20 °C 2.1: nBuLi 2.2: 89 percent / tetrahydrofuran / 0 °C 3.1: nBuLi 3.2: 95 percent / tetrahydrofuran / 0 °C 4.1: 100 percent / CSA; MeOH / 60 °C 5.1: 100 percent / DMAP; Et3N / dimethylformamide / 20 °C 6.1: 78 percent / DIBAL-H / toluene / -78 °C 7.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 8.1: 65 percent / NaH / CH2Cl2 / 20 °C 9.1: 100 percent / TBAF / tetrahydrofuran / 0 °C 10.1: 98 percent / K2CO3 / dimethylformamide / 20 °C 11.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 12.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 13.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 14.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 14.2: 43 percent / silica gel View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: 84 percent / Br2 / CHCl3 / 20 °C 2.1: 80 percent / K2CO3 / dimethylformamide / 20 °C 3.1: 73 percent / 160 °C 4.1: 87 percent / K2CO3 / dimethylformamide / 20 °C 5.1: 95 percent / OsO4; NaIO4 / H2O; diethyl ether / 20 °C 6.1: 72 percent / nBuLi / tetrahydrofuran / 0 °C 7.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 8.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 9.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 10.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 10.2: 43 percent / silica gel View Scheme |
-
-
13246-46-3
2,4-dibenzyloxybenzaldehyde

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: mCPBA / CH2Cl2 / 20 °C 2.1: HCl / methanol / 20 °C 3.1: 84 percent / Br2 / CHCl3 / 20 °C 4.1: 80 percent / K2CO3 / dimethylformamide / 20 °C 5.1: 73 percent / 160 °C 6.1: 87 percent / K2CO3 / dimethylformamide / 20 °C 7.1: 95 percent / OsO4; NaIO4 / H2O; diethyl ether / 20 °C 8.1: 72 percent / nBuLi / tetrahydrofuran / 0 °C 9.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 10.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 11.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 12.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 12.2: 43 percent / silica gel View Scheme |
-
-
120677-47-6
1,3,5-tris(methoxymethoxy)benzene

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: nBuLi 1.2: 89 percent / tetrahydrofuran / 0 °C 2.1: nBuLi 2.2: 95 percent / tetrahydrofuran / 0 °C 3.1: 100 percent / CSA; MeOH / 60 °C 4.1: 100 percent / DMAP; Et3N / dimethylformamide / 20 °C 5.1: 78 percent / DIBAL-H / toluene / -78 °C 6.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 7.1: 65 percent / NaH / CH2Cl2 / 20 °C 8.1: 100 percent / TBAF / tetrahydrofuran / 0 °C 9.1: 98 percent / K2CO3 / dimethylformamide / 20 °C 10.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 11.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 12.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 13.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 13.2: 43 percent / silica gel View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: 96 percent / K2CO3 / dimethylformamide / 20 °C 2.1: mCPBA / CH2Cl2 / 20 °C 3.1: HCl / methanol / 20 °C 4.1: 84 percent / Br2 / CHCl3 / 20 °C 5.1: 80 percent / K2CO3 / dimethylformamide / 20 °C 6.1: 73 percent / 160 °C 7.1: 87 percent / K2CO3 / dimethylformamide / 20 °C 8.1: 95 percent / OsO4; NaIO4 / H2O; diethyl ether / 20 °C 9.1: 72 percent / nBuLi / tetrahydrofuran / 0 °C 10.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 11.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 12.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 13.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 13.2: 43 percent / silica gel View Scheme |
-
-
426820-54-4
4,6-dihydroxy-2-methoxymethoxy-3-(3-methyl-but-2-enyl)-benzaldehyde

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: 98 percent / K2CO3 / dimethylformamide / 20 °C 2.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 3.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 4.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 5.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 5.2: 43 percent / silica gel View Scheme |

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: HCl / methanol / 20 °C 2.1: 84 percent / Br2 / CHCl3 / 20 °C 3.1: 80 percent / K2CO3 / dimethylformamide / 20 °C 4.1: 73 percent / 160 °C 5.1: 87 percent / K2CO3 / dimethylformamide / 20 °C 6.1: 95 percent / OsO4; NaIO4 / H2O; diethyl ether / 20 °C 7.1: 72 percent / nBuLi / tetrahydrofuran / 0 °C 8.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 9.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 10.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 11.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 11.2: 43 percent / silica gel View Scheme |
-
-
426820-42-0
1,3,5-tris-methoxymethoxy-2-(3-methyl-but-2-enyl)-benzene

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: nBuLi 1.2: 95 percent / tetrahydrofuran / 0 °C 2.1: 100 percent / CSA; MeOH / 60 °C 3.1: 100 percent / DMAP; Et3N / dimethylformamide / 20 °C 4.1: 78 percent / DIBAL-H / toluene / -78 °C 5.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 6.1: 65 percent / NaH / CH2Cl2 / 20 °C 7.1: 100 percent / TBAF / tetrahydrofuran / 0 °C 8.1: 98 percent / K2CO3 / dimethylformamide / 20 °C 9.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 10.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 11.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 12.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 12.2: 43 percent / silica gel View Scheme |

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: 100 percent / DMAP; Et3N / dimethylformamide / 20 °C 2.1: 78 percent / DIBAL-H / toluene / -78 °C 3.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 4.1: 65 percent / NaH / CH2Cl2 / 20 °C 5.1: 100 percent / TBAF / tetrahydrofuran / 0 °C 6.1: 98 percent / K2CO3 / dimethylformamide / 20 °C 7.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 8.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 9.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 10.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 10.2: 43 percent / silica gel View Scheme |
-
-
426820-51-1
2,4-bis-benzyloxy-5-bromo-phenol

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: 80 percent / K2CO3 / dimethylformamide / 20 °C 2.1: 73 percent / 160 °C 3.1: 87 percent / K2CO3 / dimethylformamide / 20 °C 4.1: 95 percent / OsO4; NaIO4 / H2O; diethyl ether / 20 °C 5.1: 72 percent / nBuLi / tetrahydrofuran / 0 °C 6.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 7.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 8.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 9.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 9.2: 43 percent / silica gel View Scheme |
-
-
426820-39-5
1-Allyloxy-2,4-bis-benzyloxy-5-bromo-benzene

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: 73 percent / 160 °C 2.1: 87 percent / K2CO3 / dimethylformamide / 20 °C 3.1: 95 percent / OsO4; NaIO4 / H2O; diethyl ether / 20 °C 4.1: 72 percent / nBuLi / tetrahydrofuran / 0 °C 5.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 6.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 7.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 8.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 8.2: 43 percent / silica gel View Scheme |
-
-
426820-52-2
(3,5-bis-benzyloxy-2-bromo-6-methoxy-phenyl)-acetaldehyde

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: 72 percent / nBuLi / tetrahydrofuran / 0 °C 2.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 3.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 4.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 5.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 5.2: 43 percent / silica gel View Scheme |
-
-
426820-56-6
2-allyl-4,6-bis-benzyloxy-3-bromo-phenol

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: 87 percent / K2CO3 / dimethylformamide / 20 °C 2.1: 95 percent / OsO4; NaIO4 / H2O; diethyl ether / 20 °C 3.1: 72 percent / nBuLi / tetrahydrofuran / 0 °C 4.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 5.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 6.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 7.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 7.2: 43 percent / silica gel View Scheme |
-
-
426820-40-8
3-Allyl-1,5-bis-benzyloxy-2-bromo-4-methoxy-benzene

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 95 percent / OsO4; NaIO4 / H2O; diethyl ether / 20 °C 2.1: 72 percent / nBuLi / tetrahydrofuran / 0 °C 3.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 4.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 5.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 6.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 6.2: 43 percent / silica gel View Scheme |
-
-
426820-43-1
2,4,6-tris-methoxymethoxy-3-(3-methyl-but-2-enyl)-benzoic acid ethyl ester

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: 100 percent / CSA; MeOH / 60 °C 2.1: 100 percent / DMAP; Et3N / dimethylformamide / 20 °C 3.1: 78 percent / DIBAL-H / toluene / -78 °C 4.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 5.1: 65 percent / NaH / CH2Cl2 / 20 °C 6.1: 100 percent / TBAF / tetrahydrofuran / 0 °C 7.1: 98 percent / K2CO3 / dimethylformamide / 20 °C 8.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 9.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 10.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 11.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 11.2: 43 percent / silica gel View Scheme |
-
-
426820-41-9
1,5-Bis-benzyloxy-2-bromo-4-methoxy-3-(3-methyl-but-2-enyl)-benzene

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 2.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 3.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 4.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 4.2: 43 percent / silica gel View Scheme |
-
-
426820-47-5
4,6-bis-benzyloxy-2-methoxymethoxy-3-(3-methyl-but-2-enyl)-benzaldehyde

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 2.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 3.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 4.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 4.2: 43 percent / silica gel View Scheme |
-
-
426820-45-3
4,6-bis-(tert-butyl-dimethyl-silanyloxy)-2-hydroxy-3-(3-methyl-but-2-enyl)-benzaldehyde

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: 65 percent / NaH / CH2Cl2 / 20 °C 2.1: 100 percent / TBAF / tetrahydrofuran / 0 °C 3.1: 98 percent / K2CO3 / dimethylformamide / 20 °C 4.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 5.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 6.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 7.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 7.2: 43 percent / silica gel View Scheme |
-
-
426820-46-4
4,6-bis-(tert-butyl-dimethyl-silanyloxy)-2-methoxymethoxy-3-(3-methyl-but-2-enyl)-benzaldehyde

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 100 percent / TBAF / tetrahydrofuran / 0 °C 2.1: 98 percent / K2CO3 / dimethylformamide / 20 °C 3.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 4.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 5.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 6.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 6.2: 43 percent / silica gel View Scheme |
-
-
426820-44-2
[2,4,6-tris-(tert-butyl-dimethyl-silanyloxy)-3-(3-methyl-but-2-enyl)-phenyl]-methanol

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 2.1: 65 percent / NaH / CH2Cl2 / 20 °C 3.1: 100 percent / TBAF / tetrahydrofuran / 0 °C 4.1: 98 percent / K2CO3 / dimethylformamide / 20 °C 5.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 6.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 7.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 8.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 8.2: 43 percent / silica gel View Scheme |

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: 78 percent / DIBAL-H / toluene / -78 °C 2.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 3.1: 65 percent / NaH / CH2Cl2 / 20 °C 4.1: 100 percent / TBAF / tetrahydrofuran / 0 °C 5.1: 98 percent / K2CO3 / dimethylformamide / 20 °C 6.1: 49 percent / sBuLi / tetrahydrofuran / -78 °C 7.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 8.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 9.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 9.2: 43 percent / silica gel View Scheme |
-
-
426820-55-5
[4,6-bis-benzyloxy-2-methoxymethoxy-3-(3-methyl-but-2-enyl)-phenyl]-[4,6-bis-benzyloxy-3-methoxy-2-(3-methyl-but-2-enyl)-phenyl]-methanone

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 2.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 2.2: 43 percent / silica gel View Scheme |
-
-
426820-48-6
[4,6-bis-benzyloxy-2-methoxymethoxy-3-(3-methyl-but-2-enyl)-phenyl]-[4,6-bis-benzyloxy-3-methoxy-2-(3-methyl-but-2-enyl)-phenyl]-methanol

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 76 percent / IBX / toluene; dimethylsulfoxide / 20 °C 2.1: 63 percent / HCO2NH4 / Pd/C / acetone / 20 °C 3.1: PPh3; CCl4 / tetrahydrofuran / 20 °C 3.2: 43 percent / silica gel View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: pyridine / 2 h / 100 °C 2: POCl3/Py / Ambient temperature 3: 5percent methanolic KOH View Scheme |
-
-
6147-11-1
α-mangostin

-
-
182317-42-6
1,3,6-trihydroxy-2,8-diisopentyl-7-methoxy-9H-xanthene-9-one

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol for 12h; | 99% |
| With palladium 10% on activated carbon; hydrogen In methanol for 12h; | 99% |
| With palladium on activated charcoal; hydrogen In methanol for 6h; | 98% |
-
-
6147-11-1
α-mangostin

-
-
108-24-7
acetic anhydride

-
-
31271-10-0
6,8-dihydroxy-2-methoxy-1,7-bis(3-methylbut-2-en-1-yl)-9-oxo-9H-xanthen-3-yl acetate

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 20℃; for 2h; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 40℃; for 3.5h; | 47.3% |

| Conditions | Yield |
|---|---|
| In benzene Mannich Aminomethylation; Reflux; | 94% |

-
-
6147-11-1
α-mangostin

-
-
19275-46-8
3,4-dihydro-5,9-dihydroxy-8-methoxy-2,2-dimethyl-7-(3-methyl-2-butenyl)-2H,6H-pyrano[3,2-b]xanthen-6-one

| Conditions | Yield |
|---|---|
| With polyphosphoric acid; air In N,N-dimethyl-formamide at 130℃; for 2h; | 91% |
| With toluene-4-sulfonic acid In benzene Reflux; | 70% |
| With toluene-4-sulfonic acid In benzene Reflux; | 70% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Reflux; | 89% |
| With potassium carbonate In acetone for 20h; Reflux; | 75% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 85℃; for 5h; | 87% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 20℃; | 85.3% |
-
-
6147-11-1
α-mangostin

-
-
35349-68-9
9-hydroxycalabaxanthone

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene at 40℃; for 5h; | 85% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In toluene at 40℃; for 5h; | 85% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In benzene at 80℃; for 3h; | 82% |
-
-
6147-11-1
α-mangostin

-
-
108-24-7
acetic anhydride

-
A
-
31271-10-0
6,8-dihydroxy-2-methoxy-1,7-bis(3-methylbut-2-en-1-yl)-9-oxo-9H-xanthen-3-yl acetate

-
B
-
107389-90-2
1,3,6-tri-O-acetyl-α-mangostin

| Conditions | Yield |
|---|---|
| With pyridine at 70℃; for 2h; | A 84.3% B 12.4% |
| With dmap In dichloromethane at 20℃; for 24h; Cooling; |


| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 85℃; for 5h; | 83% |
-
-
6147-11-1
α-mangostin

-
-
108-24-7
acetic anhydride

-
B
-
31271-10-0
6,8-dihydroxy-2-methoxy-1,7-bis(3-methylbut-2-en-1-yl)-9-oxo-9H-xanthen-3-yl acetate

| Conditions | Yield |
|---|---|
| With pyridine at 60℃; for 1h; | A 11% B 81% |

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Reflux; | 81% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Reflux; | 80% |
| With potassium carbonate In acetone at 40℃; for 4h; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 60 - 65℃; for 24h; | 80% |
| With potassium carbonate In acetone at 40℃; for 4h; |

-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Reflux; | 78% |
-
-
6147-11-1
α-mangostin

| Conditions | Yield |
|---|---|
| With osmium(VIII) oxide; 4-methylmorpholine N-oxide In water; acetone; tert-butyl alcohol at 20℃; for 24h; | A 12% B 10% C 78% |
| With osmium(VIII) oxide; water; 4-methylmorpholine N-oxide In acetone; tert-butyl alcohol at 20℃; for 24h; | A 12% B 10% C 78% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 65℃; for 24h; | 78% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Reflux; | 76.5% |
| With potassium carbonate In acetone for 24h; Reflux; | |
| With potassium carbonate In acetone for 24h; Reflux; |

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-dibromo-butane; α-mangostin With potassium carbonate In acetone for 24h; Reflux; Stage #2: 4-hydroxybenzenesulphonamide | 76.5% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 24h; | 76% |
| With potassium carbonate In acetone at 65℃; for 12h; | 15% |
| With potassium carbonate In acetone at 40℃; for 4h; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane at 20℃; for 24h; | 76% |

| Conditions | Yield |
|---|---|
| In benzene Mannich Aminomethylation; Reflux; | 75% |


| Conditions | Yield |
|---|---|
| Stage #1: α-mangostin; allyl bromide With potassium carbonate In acetone at 65℃; for 24h; Stage #2: With palladium on activated charcoal; hydrogen In methanol at 20℃; | 75% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 60 - 65℃; for 24h; | 75% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Reflux; | 73% |

| Conditions | Yield |
|---|---|
| In benzene at 20℃; for 0.75h; Mannich Aminomethylation; | 73% |
-
-
6147-11-1
α-mangostin

-
A
-
19275-46-8
3,4-dihydro-5,9-dihydroxy-8-methoxy-2,2-dimethyl-7-(3-methyl-2-butenyl)-2H,6H-pyrano[3,2-b]xanthen-6-one

-
B
-
19275-44-6
1-isomangostin

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 0.5h; Reflux; Dean-Stark; | A 65% B 15% |
-
-
6147-11-1
α-mangostin

-
A
-
26063-95-6
1-isomangostin hydrate

-
B
-
19275-46-8
3,4-dihydro-5,9-dihydroxy-8-methoxy-2,2-dimethyl-7-(3-methyl-2-butenyl)-2H,6H-pyrano[3,2-b]xanthen-6-one

| Conditions | Yield |
|---|---|
| With sulfuric acid In toluene at 40℃; for 4h; Inert atmosphere; Molecular sieve; | A 63% B 29% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Reflux; | 62% |
| With potassium carbonate In acetone for 24h; Reflux; | 62% |
Mangostin Chemical Properties
Molecular Structure of Mangostin (6147-11-1):
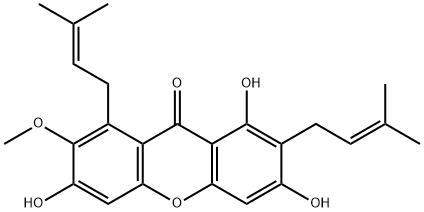
IUPAC Name: 1,3,6-Trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-enyl)xanthen-9-one
Molecular Formula: C24H26O6
Molecular Weight: 410.45964 g/mol
XLogP3-AA: 6.3
H-Bond Donor: 3
H-Bond Acceptor: 6
Canonical SMILES: CC(=CCC1=C(C=C2C(=C1O)C(=O)C3=C(C(=C(C=C3O2)O)OC)CC=C(C)C)O)C
InChI: InChI=1S/C24H26O6/c1-12(2)6-8-14-16(25)10-19-21(22(14)27)23(28)20-15(9-7-13(3)4)24(29-5)17(26)11-18(20)30-19/h6-7,10-11,25-27H,8-9H2,1-5H3
InChIKey: GNRIZKKCNOBBMO-UHFFFAOYSA-N
Index of Refraction: 1.624
Molar Refractivity: 114.55 cm3
Molar Volume: 324.4 cm3
Surface Tension: 53.9 dyne/cm
Density: 1.265 g/cm3
Flash Point: 220.3 °C
Melting Point: 180-182 °C
Boiling Point: 640.1 °C at 760 mmHg
Enthalpy of Vaporization: 97.94 kJ/mol
Vapour Pressure: 5.59E-17 mmHg at 25 °C
Water Solubility: 0.0002026 mg/L at 25 °C
Mangostin Specification
Mangostin (6147-11-1) is an anti-inflammatory agent which is isolated from Garcinia mangostana Linn. Mangostin (6147-11-1) is also known as alpha-Mangostin ; 1,3,6-Trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-9H-xanthen-9-one ; 9H-Xanthen-9-one, 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)- (9CI) ; Xanthen-9-one, 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-(8CI) ; 1,3,6-Trihydroxy-7-methoxy-2,8-bis(3-methyl-2-buten-1-yl)-9H-xanthen-9-on ; 1,3,6-Trihydroxy-7-methoxy-2,8-bis(3-methyl-2-buten-1-yl)-9H-xanthen-9-one ; 1,3,6-Trihydroxy-7-méthoxy-2,8-bis(3-méthyl-2-butèn-1-yl)-9H-xanthén-9-one ; 1,3,6-Trihydroxy-7-methoxy-2,8-di(3-methyl-2-butenyl)xanthone ; 9H-Xanthen-9-one, 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-buten-1-yl)- ; 9H-Xanthen-9-one, 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)- with appearance of yellow crystalline solid.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View