-
Name
Methanesulfonamide
- EINECS 221-553-6
- CAS No. 3144-09-0
- Article Data79
- CAS DataBase
- Density 1.363 g/cm3
- Solubility
- Melting Point 85-89 °C(lit.)
- Formula CH5NO2S
- Boiling Point 208.2 °C at 760 mmHg
- Molecular Weight 95.1222
- Flash Point 79.7 °C
- Transport Information
- Appearance white to yellow crystals, powder or flakes
- Safety 26-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Mesylamide;Methanesulfonic amide;Methanesulphonamide;NSC 191039;NSC271;
- PSA 68.54000
- LogP 0.68580
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonia In dichloromethane for 0.0833333h; | A n/a B 100% |
-
-
1578267-78-3
N-(3-methylbut-2-en-1-yl)methanesulfonamide

-
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate In 1,2-dichloro-ethane at 140℃; for 0.0333333h; Inert atmosphere; Microwave irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; ethyl acetate | 99% |
| With triethylamine In dichloromethane | 98% |
| 97% |

-
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate In 1,2-dichloro-ethane at 140℃; for 0.0333333h; Microwave irradiation; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; water | 95.6% |
| With triethylamine In dichloromethane; water | 95.6% |
| With triethylamine In dichloromethane; water |

-
-
624-90-8, 1516-70-7
Methanesulfonyl azide

-
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| With benzyltriethylammonium tetrathiomolybdate In water; acetonitrile at 25℃; for 0.1h; | 95% |
| With zinc In methanol at 20℃; for 2h; | 92% |
| With iron In water at 20℃; for 2h; Inert atmosphere; | 87% |

-
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate In 1,2-dichloro-ethane at 140℃; for 0.0333333h; Microwave irradiation; Inert atmosphere; | 81% |
-
-
31562-43-3
tert-butylsulfinyl chloride

-
-
50695-55-1
N-hydroxylmethanesulfonamide

-
A
-
3144-09-0
methanesulfonamide

-
B
-
31562-41-1
di-tert-butyl thiosulfonate

-
C
-
75975-45-0
trifluoromethane sulfonic anhydride

-
D
-
75975-41-6
C5H13NO4S2

| Conditions | Yield |
|---|---|
| With pyridine In acetone at 20℃; for 2h; Mechanism; Product distribution; other N-hydroxy sulfonamides; | A 80% B 13% C 22% D 15% |


-
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol at 20℃; for 2h; | 80% |
-
-
362665-07-4
N-methanesulfonyl-3-phenylpropylamine

-
A
-
104-53-0
3-phenyl-propionaldehyde

-
B
-
3144-09-0
methanesulfonamide

-
C
-
213264-51-8
3-(4-iodophenyl)propionaldehyde

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; iodine In 1,2-dichloro-ethane at 30 - 40℃; for 3h; ultrasonic irradiation; | A 24% B 66% C 39% |


| Conditions | Yield |
|---|---|
| With water for 1h; Heating; | A 56% B n/a |
-
-
303165-07-3
4-{2-[3,4-bis(difluoromethoxy)phenyl]-2-[6-(4-aminophenoxy)-pyridin-3-yl]ethyl}pyridine

-
-
124-63-0
methanesulfonyl chloride


-
B
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| With potassium carbonate; triethylamine In tetrahydrofuran; methanol; dichloromethane | A 51% B n/a |

-
-
20277-69-4
sodium methansulfinate

-
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: sodium methansulfinate With iodine at 20℃; for 0.333333h; Green chemistry; Stage #2: With ammonium hydroxide; water at 20℃; for 3h; Green chemistry; | 23% |

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide | A n/a B 21% |

-
-
292638-84-7
styrene

-
A
-
3144-09-0
methanesulfonamide

-
B
-
102535-89-7
(N-methanesulfonyl)-2-phenylaziridine

| Conditions | Yield |
|---|---|
| With copper acetylacetonate In acetonitrile Ambient temperature; | A n/a B 7% |

-
-
859980-70-4
N,N-bis-methanesulfonyl-glycine

-
-
623-11-0
1-methyl-4-nitrosobenzene

-
A
-
79-14-1
glycolic Acid

-
B
-
3144-09-0
methanesulfonamide

-
C
-
35688-18-7
2-(methylsulfonylamino)acetic acid


| Conditions | Yield |
|---|---|
| With water |

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction. Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With acidic media Product distribution; heating; |

| Conditions | Yield |
|---|---|
| With acidic media Product distribution; heating; |
-
-
75142-05-1
Trimesylhydroxylamine

-
A
-
3144-09-0
methanesulfonamide

-
B
-
5347-82-0
dimesylamine

-
C
-
2386-57-4
methanesulfonic acid sodium salt

-
D
-
41921-91-9
N-methylsulfamic acid monosodium salt

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Mechanism; Product distribution; Heating; hydrolysis with basic, neutral and acidic aqu. solutions (other products); | A 6 % Spectr. B 20 % Spectr. C 70 % Spectr. D 4 % Spectr. |


-
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| With water In methanol Mechanism; |
-
-
41138-92-5
methanesulfonyl bromide

-
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| With ammonia; triethylamine In acetonitrile at 0 - 20℃; Yield given; |
-
-
3611-92-5
methanesulfonyl isocyanate

-
-
64-17-5
ethanol

-
A
-
3144-09-0
methanesulfonamide

-
B
-
124-38-9
carbon dioxide

-
-
3611-92-5
methanesulfonyl isocyanate

-
-
7732-18-5
water

-
A
-
3144-09-0
methanesulfonamide

-
B
-
124-38-9
carbon dioxide


| Conditions | Yield |
|---|---|
| With water |
-
-
3144-09-0
methanesulfonamide

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
147751-16-4
tert-butyl (methylsulfonyl)carbamate

| Conditions | Yield |
|---|---|
| With trimethylamine In dichloromethane at 0 - 20℃; for 16h; | 100% |
| With triethylamine; dmap In dichloromethane for 2h; Ambient temperature; | 88% |
| With triethylamine; dmap In dichloromethane at 25℃; for 2h; | 83% |

| Conditions | Yield |
|---|---|
| With phenformin In dichloromethane at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With phenformin In dichloromethane at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 12h; | 100% |
-
-
3144-09-0
methanesulfonamide

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
78523-02-1
N-[1-Dimethylamino-meth-(E)-ylidene]-methanesulfonamide

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.5h; Heating; | 100% |
-
-
3144-09-0
methanesulfonamide

-
-
106-38-7
para-bromotoluene

-
-
4284-47-3
N-(4-methylphenyl)methanesulfonamide

| Conditions | Yield |
|---|---|
| With potassium phosphate; tris-(dibenzylideneacetone)dipalladium(0); tert-butyl XPhos In tert-Amyl alcohol at 80℃; Kinetics; Inert atmosphere; | 100% |
| With bis(η3-allyl-μ-chloropalladium(II)); potassium carbonate; tert-butyl XPhos In 2-methyltetrahydrofuran at 80℃; for 6h; Inert atmosphere; | 93% |
| With potassium phosphate; copper(l) iodide; dimethylaminoacetic acid In N,N-dimethyl-formamide for 48h; Heating; | 89% |

| Conditions | Yield |
|---|---|
| With dmap; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In dichloromethane at 20℃; | 100% |
-
-
3144-09-0
methanesulfonamide

-
-
1064681-32-8
1-(3-Iodophenyl)-2-methoxy-6-(3-methoxyphenyl)naphthalene

-
-
13939-06-5, 199620-15-0
molybdenum hexacarbonyl

-
-
1064681-28-2
3-(2-Methoxy-6-(3-methoxyphenyl)naphthalene-1-yl)-N-(methylsulfonyl)-benzamide

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; palladium diacetate In 1,4-dioxane at 110℃; for 0.25h; microwave irradiation; | 100% |

-
-
3144-09-0
methanesulfonamide

-
-
1354960-59-0
4-Methylphenyl 5-chloro-4-(3,4-dichlorophenoxy)-2-fluorobenzoate

-
-
1354955-37-5
5-Chloro-4-(3,4-dichlorophenoxy)-2-fluoro-N-(methylsulfonyl)benzamide

| Conditions | Yield |
|---|---|
| Stage #1: methanesulfonamide With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 0.516667h; Stage #2: 4-Methylphenyl 5-chloro-4-(3,4-dichlorophenoxy)-2-fluorobenzoate In tetrahydrofuran; mineral oil at 70℃; | 100% |
-
-
497225-66-8
2-n-butyl-5-bromo-benzofuran

-
-
3144-09-0
methanesulfonamide

-
-
437652-07-8
N-(2-butyl-2,3-dihydro-5-benzofuranyl)methanesulfonamide

| Conditions | Yield |
|---|---|
| With bis(dibenzylideneacetone)-palladium(0); tert-butyl XPhos In 1,4-dioxane at 100℃; for 24h; Reagent/catalyst; Solvent; Inert atmosphere; | 100% |
-
-
3144-09-0
methanesulfonamide

-
-
1445862-88-3
4-(4-chlorophenoxy)-2-fluoro-5-iodobenzoic acid

-
-
1445862-89-4
4-(4-chlorophenoxy)-2-fluoro-5-iodo-N-(methylsulfonyl)benzamide

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 100% |
-
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 100% |
-
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl acetamide at 80℃; for 11h; | 100% |
-
-
3144-09-0
methanesulfonamide

-
-
461009-54-1
(S)-3-(((benzyloxy)carbonyl)amino)-4-((tert-butyldiphenylsilyl)oxy)butanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (S)-3-(((benzyloxy)carbonyl)amino)-4-((tert-butyldiphenylsilyl)oxy)butanoic acid With 1,1'-carbonyldiimidazole at 20℃; for 3h; Inert atmosphere; Stage #2: methanesulfonamide With 1,8-diazabicyclo[5.4.0]undec-7-ene at 20℃; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; dimethylaminoacetic acid In N,N-dimethyl-formamide for 48h; Heating; | 99% |
| With potassium phosphate; copper(l) iodide In N,N-dimethyl-formamide at 130℃; for 18h; Sealed tube; | 75% |
| With copper(l) iodide; potassium carbonate In various solvent(s) at 195℃; for 2h; microwave irradiation; | 67% |
| With copper(I) oxide; caesium carbonate In water at 130℃; for 24h; Sealed tube; | 36% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; sarcosine In N,N-dimethyl-formamide at 100℃; for 24h; | 99% |
| With copper(I) oxide; caesium carbonate In water at 130℃; for 24h; Catalytic behavior; Reagent/catalyst; Solvent; Sealed tube; | 90% |
| With copper(I) oxide; caesium carbonate In water at 130℃; for 24h; Sealed tube; | 90% |
-
-
3144-09-0
methanesulfonamide

-
-
618-91-7
methyl 3-iodo-benzoate

-
-
32087-05-1
3-methanesulfonylaminobenzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; sarcosine In N,N-dimethyl-formamide at 100℃; for 24h; | 99% |
-
-
3144-09-0
methanesulfonamide

-
-
177896-09-2
tert-butyl benzylidenecarbamate

| Conditions | Yield |
|---|---|
| With bis(trifluoromethanesulfonyl)amide In diethyl ether at 20℃; for 0.333333h; | 99% |
-
-
3144-09-0
methanesulfonamide

-
-
26825-94-5
10-bromodecanoic acid methyl ester

-
-
1161364-44-8
methyl 10-(methylsulfonamido)decanoate

| Conditions | Yield |
|---|---|
| Stage #1: methanesulfonamide With sodium hydride In N,N-dimethyl-formamide at 100℃; for 2h; Stage #2: 10-bromodecanoic acid methyl ester In N,N-dimethyl-formamide at 100℃; | 99% |
-
-
3144-09-0
methanesulfonamide

-
-
62668-02-4
(E)-1,3-diphenyl-2-propen-1-ol

-
-
1262765-49-0
N-[(2E)-1,3-diphenyl-2-propen-1-yl]methanesulfonamide

| Conditions | Yield |
|---|---|
| With 3-tetradecyl-1-(4-sulfobutyl)imidazolium trifluoromethanesulfonate In 1,4-dioxane at 80℃; for 2h; | 99% |
| With [BsTdmim]OTf In 1,4-dioxane at 80℃; for 2h; | 99% |
| With iron(III) chloride hexahydrate In 1,4-dioxane at 25℃; for 24h; | 89% |

-
-
3144-09-0
methanesulfonamide

| Conditions | Yield |
|---|---|
| In dichloromethane 23°C, 0.5-1 min; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In butanone at 75℃; | 99% |
| With potassium carbonate; potassium iodide In butanone at 81℃; for 18h; Concentration; Temperature; | 81% |
| Stage #1: methanesulfonamide With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 5h; Stage #2: p-methoxybenzyl chloride In N,N-dimethyl-formamide at 20℃; for 19h; | 17.1 g |
| With potassium carbonate; potassium iodide In butanone at 75℃; |
-
-
188813-02-7
3-bromo-5-fluorobenzaldehyde

-
-
3144-09-0
methanesulfonamide

-
-
1467083-70-0
N-(3-bromo-5-fluorobenzyl) methanesulfonamide

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride; triethylamine In 1,2-dichloro-ethane at 20℃; | 99% |
| With sodium tris(acetoxy)borohydride; triethylamine In 1,2-dichloro-ethane at 20℃; | 93.9% |
-
-
3144-09-0
methanesulfonamide

-
-
207743-78-0
tert-butyl 2-isothiocyanato-4,5,6,7-tetrahydrobenzo[b]-thiophene-3-carboxylate

-
-
1452776-84-9
tert-butyl 2-(3-(methylsulfonyl)thioureido)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: methanesulfonamide With sodium hydride In N,N-dimethyl-formamide; mineral oil for 0.25h; Stage #2: tert-butyl 2-isothiocyanato-4,5,6,7-tetrahydrobenzo[b]-thiophene-3-carboxylate In N,N-dimethyl-formamide; mineral oil at 60℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| With dmap for 72h; | 99% |
-
-
3144-09-0
methanesulfonamide

-
-
415965-24-1
4-bromo-2-fluoro-5-methylbenzoic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 99% |
-
-
3144-09-0
methanesulfonamide


| Conditions | Yield |
|---|---|
| Stage #1: 2-(5-((S)-1-((tert-butoxycarbonyl)amino)ethyl)-2-(3-(cyclopropylmethoxy)-4-(difluoromethoxy)phenyl)oxazole-4-carboxamido)-2-(2,4-difluorophenyl)acetic acid With 1,1'-carbonyldiimidazole In tetrahydrofuran at 60℃; for 0.5h; Stage #2: methanesulfonamide With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 60℃; for 10h; | 99% |
Methanesulfonamide Chemical Properties
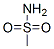
Formula Weight: 95.12
Synonyms: Methylsulfonamid; Methanesulfonic amide; Methanesulfonyl amide; METHYLSULFAMIDE
Melting Point: 85-89 °C(lit.)
Flash Point: 79.7 °C
Boiling Point: 208.2 °C at 760 mmHg
Density of Methanesulfonamide (3144-09-0): 1.363 g/cm3
Vapour Pressure: 0.216 mmHg at 25°C
Index of Refraction: 1.46
Appearence: Methanesulfonamide (3144-09-0) is an off-white flake
Methanesulfonamide Uses
Methanesulfonamide Safety Profile
Hazard Codes : Xi: Irritant
Risk Statements : 36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements : 26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
24/25: Avoid contact with skin and eyes
WGK Germany : 3
Hazard Note : Irritant
TSCA : T
HS Code : 29350090
Methanesulfonamide Standards and Recommendations
WATER: 0.5% max
LOSS ON DRYING: 2.0% max
HEAVY METALS: 20ppm max
Methanesulfonamide Specification
Related Products
- Methanesulfonamide
- Methanesulfonamide, N-(1'-(2-(5-benzofurazanyl)ethyl)-3,4-dihydro-4-oxospiro(2H-1-benzopyran-2,4'-piperidin-6-yl)-, monohydrochloride
- Methanesulfonamide, N-(4-(2-(diethylamino)-1-oxopropyl)phenyl)-, monohydrochloride
- Methanesulfonamide, N-[5-(4,5-dihydro-1H-imidazol-2-yl)-5,6,7,8-tetrahydro-2-hydroxy-1-naphthalenyl]-,hydrobromide (1:1)
- Methanesulfonamide, N-2-propenyl-
- Methanesulfonamide,1-chloro-N-(phenylmethyl)-
- Methanesulfonamide,N-(2-aminophenyl)-
- Methanesulfonamide,N-(2-chloroethyl)-
- Methanesulfonamide,N-(2-cyanophenyl)-
- Methanesulfonamide,N-(3-formylphenyl)-
- 314-41-0
- 3144-16-9
- 31442-54-3
- 3144-54-5
- 314-46-5
- 3144-74-9
- 31448-54-1
- 314-50-1
- 31457-07-5
- 3145-76-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View